Comprehensive validation of published immunohistochemical prognostic biomarkers of prostate cancer -what has gone wrong? A blueprint for the way forward in biomarker studies
- PMID: 25422912
- PMCID: PMC4453620
- DOI: 10.1038/bjc.2014.588
Comprehensive validation of published immunohistochemical prognostic biomarkers of prostate cancer -what has gone wrong? A blueprint for the way forward in biomarker studies
Abstract
Background: Treatment planning of localised prostate cancer remains challenging. Besides conventional parameters, a wealth of prognostic biomarkers has been proposed so far. None of which, however, have successfully been implemented in a routine setting so far. The aim of our study was to systematically verify a set of published prognostic markers for prostate cancer.
Methods: Following an in-depth PubMed search, 28 markers were selected that have been proposed as multivariate prognostic markers for primary prostate cancer. Their prognostic validity was examined in a radical prostatectomy cohort of 238 patients with a median follow-up of 60 months and biochemical progression as endpoint of the analysis. Immunohistochemical evaluation was performed using previously published cut-off values, but allowing for optimisation if necessary. Univariate and multivariate Cox regression were used to determine the prognostic value of biomarkers included in this study.
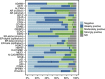
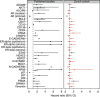
Results: Despite the application of various cut-offs in the analysis, only four (14%) markers were verified as independently prognostic (AKT1, stromal AR, EZH2, and PSMA) for PSA relapse following radical prostatectomy.
Conclusions: Apparently, many immunohistochemistry-based studies on prognostic markers seem to be over-optimistic. Codes of best practice, such as the REMARK guidelines, may facilitate the performance of conclusive and transparent future studies.
Figures

Similar articles
-
Histopathological variables and biomarkers enhancer of zeste homologue 2, Ki-67 and minichromosome maintenance protein 7 as prognosticators in primarily endocrine-treated prostate cancer.BJU Int. 2011 Nov;108(9):1430-8. doi: 10.1111/j.1464-410X.2011.10253.x. Epub 2011 May 18. BJU Int. 2011. PMID: 21592298
-
Cyclooxygenase-2 (COX-2) expression is an independent predictor of prostate cancer recurrence.Int J Cancer. 2006 Sep 1;119(5):1082-7. doi: 10.1002/ijc.21749. Int J Cancer. 2006. PMID: 16557596
-
Large-scale independent validation of the nuclear factor-kappa B p65 prognostic biomarker in prostate cancer.Eur J Cancer. 2013 Jul;49(10):2441-8. doi: 10.1016/j.ejca.2013.02.026. Epub 2013 Mar 28. Eur J Cancer. 2013. PMID: 23541563
-
New and Emerging Diagnostic and Prognostic Immunohistochemical Biomarkers in Prostate Pathology.Adv Anat Pathol. 2017 Jan;24(1):35-44. doi: 10.1097/PAP.0000000000000136. Adv Anat Pathol. 2017. PMID: 27941540 Free PMC article. Review.
-
An overview of translational prostate cancer cohorts for prognostic and predictive studies.Histopathology. 2019 Jan;74(1):161-170. doi: 10.1111/his.13770. Histopathology. 2019. PMID: 30565297 Review.
Cited by
-
1,4-dihydroxy quininib modulates the secretome of uveal melanoma tumour explants and a marker of oxidative phosphorylation in a metastatic xenograft model.Front Med (Lausanne). 2023 Jan 9;9:1036322. doi: 10.3389/fmed.2022.1036322. eCollection 2022. Front Med (Lausanne). 2023. PMID: 36698840 Free PMC article.
-
[Reports of prostate needle biopsies-what pathologists provide and urologists want].Urologe A. 2020 Apr;59(4):461-468. doi: 10.1007/s00120-020-01121-z. Urologe A. 2020. PMID: 32016505 German.
-
Prognostic role of TSPAN1, KIAA1324 and ESRP1 in prostate cancer.APMIS. 2021 Apr;129(4):204-212. doi: 10.1111/apm.13117. Epub 2021 Feb 2. APMIS. 2021. PMID: 33455017 Free PMC article.
-
Liver Microenvironment Response to Prostate Cancer Metastasis and Hormonal Therapy.Cancers (Basel). 2022 Dec 15;14(24):6189. doi: 10.3390/cancers14246189. Cancers (Basel). 2022. PMID: 36551674 Free PMC article. Review.
-
CDO1 promoter methylation is associated with gene silencing and is a prognostic biomarker for biochemical recurrence-free survival in prostate cancer patients.Epigenetics. 2016 Dec;11(12):871-880. doi: 10.1080/15592294.2016.1241931. Epub 2016 Sep 30. Epigenetics. 2016. PMID: 27689475 Free PMC article.
References
-
- Altman DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of using "optimal" cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst. 1994;86 (11:829–835. - PubMed
-
- Attard G, Clark J, Ambroisine L, Fisher G, Kovacs G, Flohr P, Berney D, Foster CS, Fletcher A, Gerald WL, Moller H, Reuter V, De Bono JS, Scardino P, Cuzick J, Cooper CS. Duplication of the fusion of TMPRSS2 to ERG sequences identifies fatal human prostate cancer. Oncogene. 2008;27 (3:253–263. - PMC - PubMed
-
- Attard G, de Bono JS. Prostate cancer: PSA as an intermediate end point in clinical trials. Nat Rev Urol. 2009;6 (9:473–475. - PubMed
-
- Beer M, Montani M, Gerhardt J, Wild PJ, Hany TF, Hermanns T, Muntener M, Kristiansen G. Profiling gastrin-releasing peptide receptor in prostate tissues: clinical implications and molecular correlates. Prostate. 2012;72 (3:318–325. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

