Live-cell mass profiling: an emerging approach in quantitative biophysics
- PMID: 25423019
- PMCID: PMC4319180
- DOI: 10.1038/nmeth.3175
Live-cell mass profiling: an emerging approach in quantitative biophysics
Abstract
Cell mass, volume and growth rate are tightly controlled biophysical parameters in cellular development and homeostasis, and pathological cell growth defines cancer in metazoans. The first measurements of cell mass were made in the 1950s, but only recently have advances in computer science and microfabrication spurred the rapid development of precision mass-quantifying approaches. Here we discuss available techniques for quantifying the mass of single live cells with an emphasis on relative features, capabilities and drawbacks for different applications.
Figures
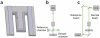
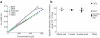

References
-
- Lloyd AC. The regulation of cell size. Cell. 2013;154:1194–1205. Recent review summarizing the current understanding of cell size control mechanisms. - PubMed
-
- O’Farrell PH. In: Cell Growth: Control of Cell Size. Hall MN, Raff M, Thomas G, editors. Cold Spring Harbor Press; 2004. pp. 1–22. Ch. 1.
-
- Mitchison JM. The growth of single cells: I. Schizosaccharomyces pombe. Exp. Cell Res. 1957;13:244–262. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 CA090571/CA/NCI NIH HHS/United States
- PN2 EY018228/EY/NEI NIH HHS/United States
- K25CA157940/CA/NCI NIH HHS/United States
- R01 CA156674/CA/NCI NIH HHS/United States
- R01CA90571/CA/NCI NIH HHS/United States
- T32 CA009120/CA/NCI NIH HHS/United States
- PN2EY018228/EY/NEI NIH HHS/United States
- P01GM081621/GM/NIGMS NIH HHS/United States
- R01 CA185189/CA/NCI NIH HHS/United States
- R01 CA107300/CA/NCI NIH HHS/United States
- R01 GM073981/GM/NIGMS NIH HHS/United States
- R01CA156674/CA/NCI NIH HHS/United States
- R01CA185189/CA/NCI NIH HHS/United States
- K25 CA157940/CA/NCI NIH HHS/United States
- P01 GM081621/GM/NIGMS NIH HHS/United States
- R01GM073981/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

