Impact of Xpert MTB/RIF for TB diagnosis in a primary care clinic with high TB and HIV prevalence in South Africa: a pragmatic randomised trial
- PMID: 25423041
- PMCID: PMC4244039
- DOI: 10.1371/journal.pmed.1001760
Impact of Xpert MTB/RIF for TB diagnosis in a primary care clinic with high TB and HIV prevalence in South Africa: a pragmatic randomised trial
Abstract
Background: Xpert MTB/RIF is approved for use in tuberculosis (TB) and rifampicin-resistance diagnosis. However, data are limited on the impact of Xpert under routine conditions in settings with high TB burden.
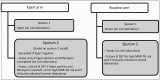
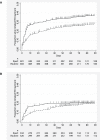
Methods and findings: A pragmatic prospective cluster-randomised trial of Xpert for all individuals with presumptive (symptomatic) TB compared to the routine diagnostic algorithm of sputum microscopy and limited use of culture was conducted in a large TB/HIV primary care clinic. The primary outcome was the proportion of bacteriologically confirmed TB cases not initiating TB treatment by 3 mo after presentation. Secondary outcomes included time to TB treatment and mortality. Unblinded randomisation occurred on a weekly basis. Xpert and smear microscopy were performed on site. Analysis was both by intention to treat (ITT) and per protocol. Between 7 September 2010 and 28 October 2011, 1,985 participants were assigned to the Xpert (n = 982) and routine (n = 1,003) diagnostic algorithms (ITT analysis); 882 received Xpert and 1,063 routine (per protocol analysis). 13% (32/257) of individuals with bacteriologically confirmed TB (smear, culture, or Xpert) did not initiate treatment by 3 mo after presentation in the Xpert arm, compared to 25% (41/167) in the routine arm (ITT analysis, risk ratio 0.51, 95% CI 0.33-0.77, p = 0.0052). The yield of bacteriologically confirmed TB cases among patients with presumptive TB was 17% (167/1,003) with routine diagnosis and 26% (257/982) with Xpert diagnosis (ITT analysis, risk ratio 1.57, 95% CI 1.32-1.87, p<0.001). This difference in diagnosis rates resulted in a higher rate of treatment initiation in the Xpert arm: 23% (229/1,003) and 28% (277/982) in the routine and Xpert arms, respectively (ITT analysis, risk ratio 1.24, 95% CI 1.06-1.44, p = 0.013). Time to treatment initiation was improved overall (ITT analysis, hazard ratio 0.76, 95% CI 0.63-0.92, p = 0.005) and among HIV-infected participants (ITT analysis, hazard ratio 0.67, 95% CI 0.53-0.85, p = 0.001). There was no difference in 6-mo mortality with Xpert versus routine diagnosis. Study limitations included incorrect intervention allocation for a high proportion of participants and that the study was conducted in a single clinic.
Conclusions: These data suggest that in this routine primary care setting, use of Xpert to diagnose TB increased the number of individuals with bacteriologically confirmed TB who were treated by 3 mo and reduced time to treatment initiation, particularly among HIV-infected participants.
Trial registration: Pan African Clinical Trials Registry PACTR201010000255244. Please see later in the article for the Editors' Summary.
Conflict of interest statement
CCB is employed by the Foundation for Innovative New Diagnostics (FIND, Geneva, Switzerland), a non-profit organisation that collaborates with industry partners, including Cepheid (Sunnyvale, CA, USA), the developer of Xpert, on the development, assessment, and demonstration of new diagnostic tests. All other authors declare no competing interests. No commercial partner was involved in the study.
Figures


References
-
- World Health Organization (2013) Global tuberculosis report 2013. WHO/HTM/TB/2013.11. Geneva: World Health Organization.
-
- Lawn SD, Ayles H, Egwaga S, Williams B, Mukadi YD, et al. (2011) Potential utility of empirical tuberculosis treatment for HIV-infected patients with advanced immunodeficiency in high TB-HIV burden settings. Int J Tuberc Lung Dis 15: 287–295. - PubMed
-
- World Health Organization (2010) Roadmap for rolling out Xpert MTB/RIF for rapid diagnosis of TB and MDR-TB. Geneva: World Health Organization.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

