Chemical-free inactivated whole influenza virus vaccine prepared by ultrashort pulsed laser treatment
- PMID: 25423046
- PMCID: PMC4242973
- DOI: 10.1117/1.JBO.20.5.051008
Chemical-free inactivated whole influenza virus vaccine prepared by ultrashort pulsed laser treatment
Abstract
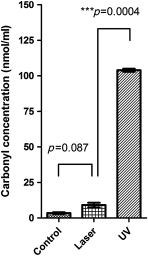
There is an urgent need for rapid methods to develop vaccines in response to emerging viral pathogens. Whole inactivated virus (WIV) vaccines represent an ideal strategy for this purpose; however, a universal method for producing safe and immunogenic inactivated vaccines is lacking. Conventional pathogen inactivation methods such as formalin, heat, ultraviolet light, and gamma rays cause structural alterations in vaccines that lead to reduced neutralizing antibody specificity, and in some cases, disastrous T helper type 2-mediated immune pathology. We have evaluated the potential of a visible ultrashort pulsed (USP) laser method to generate safe and immunogenic WIV vaccines without adjuvants. Specifically, we demonstrate that vaccination of mice with laser-inactivated H1N1 influenza virus at about a 10-fold lower dose than that required using conventional formalin-inactivated influenza vaccines results in protection against lethal H1N1 challenge in mice. The virus, inactivated by the USP laser irradiation, has been shown to retain its surface protein structure through hemagglutination assay. Unlike conventional inactivation methods, laser treatment did not generate carbonyl groups in protein, thereby reducing the risk of adverse vaccine-elicited T helper type 2 responses. Therefore, USP laser treatment is an attractive potential strategy to generate WIV vaccines with greater potency and safety than vaccines produced by current inactivation techniques.
Figures




Similar articles
-
Induction of heterosubtypic cross-protection against influenza by a whole inactivated virus vaccine: the role of viral membrane fusion activity.PLoS One. 2012;7(1):e30898. doi: 10.1371/journal.pone.0030898. Epub 2012 Jan 27. PLoS One. 2012. PMID: 22303469 Free PMC article.
-
Inactivated H7 Influenza Virus Vaccines Protect Mice despite Inducing Only Low Levels of Neutralizing Antibodies.J Virol. 2017 Sep 27;91(20):e01202-17. doi: 10.1128/JVI.01202-17. Print 2017 Oct 15. J Virol. 2017. PMID: 28768855 Free PMC article.
-
Cross-Protective Potential and Protection-Relevant Immune Mechanisms of Whole Inactivated Influenza Virus Vaccines Are Determined by Adjuvants and Route of Immunization.Front Immunol. 2019 Mar 29;10:646. doi: 10.3389/fimmu.2019.00646. eCollection 2019. Front Immunol. 2019. PMID: 30984200 Free PMC article.
-
Inactivation methods for whole influenza vaccine production.Rev Med Virol. 2019 Nov;29(6):e2074. doi: 10.1002/rmv.2074. Epub 2019 Jul 23. Rev Med Virol. 2019. PMID: 31334909 Review.
-
Return of inactivated whole-virus vaccine for superior efficacy.Immunol Cell Biol. 2012 Jul;90(6):571-8. doi: 10.1038/icb.2011.70. Epub 2011 Aug 16. Immunol Cell Biol. 2012. PMID: 21844883 Review.
Cited by
-
Selective photonic disinfection of cell culture using a visible ultrashort pulsed laser.IEEE J Sel Top Quantum Electron. 2016 May-Jun;22(3):7100508. doi: 10.1109/JSTQE.2015.2498920. Epub 2015 Nov 9. IEEE J Sel Top Quantum Electron. 2016. PMID: 27013847 Free PMC article.
-
Viral inactivation by light.eLight. 2022;2(1):18. doi: 10.1186/s43593-022-00029-9. Epub 2022 Sep 26. eLight. 2022. PMID: 36187558 Free PMC article. Review.
-
An Overview of Veterinary Viral Diseases and Vaccine Technologies.Methods Mol Biol. 2022;2465:1-26. doi: 10.1007/978-1-0716-2168-4_1. Methods Mol Biol. 2022. PMID: 35118613 Review.
-
Phototherapy as a Rational Antioxidant Treatment Modality in COVID-19 Management; New Concept and Strategic Approach: Critical Review.Antioxidants (Basel). 2020 Sep 16;9(9):875. doi: 10.3390/antiox9090875. Antioxidants (Basel). 2020. PMID: 32947974 Free PMC article. Review.
-
Inactivation of multidrug-resistant bacteria and bacterial spores and generation of high-potency bacterial vaccines using ultrashort pulsed lasers.J Biophotonics. 2022 Feb;15(2):e202100207. doi: 10.1002/jbio.202100207. Epub 2021 Dec 8. J Biophotonics. 2022. PMID: 34802194 Free PMC article.
References
-
- Mocarski E. S., Shenk T., Pass R. F., Fields Virology, pp. 2701–2772, Lippincott Williams and Wilkins, Philadelphia, Pennsylvania: (2007).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

