Enantioselective synthesis of spliceostatin E and evaluation of biological activity
- PMID: 25423085
- PMCID: PMC4260646
- DOI: 10.1021/ol503127r
Enantioselective synthesis of spliceostatin E and evaluation of biological activity
Abstract
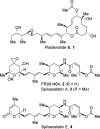
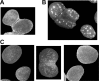
An enantioselective total synthesis of spliceostatin E has been accomplished. The δ-lactone unit A was constructed from readily available (R)-glycidyl alcohol using a ring-closing olefin metathesis as the key reaction. A cross-metathesis of ring A containing δ-lactone and the functionalized tetrahydropyran B-ring provided spliceostatin E. Our biological evaluation of synthetic spliceostatin E revealed that it does not inhibit splicing in vitro and does not impact speckle morphology in cells. Spliceostatin E was reported to possess potent antitumor activity.
Figures






Similar articles
-
Enantioselective Synthesis of a Cyclopropane Derivative of Spliceostatin A and Evaluation of Bioactivity.Org Lett. 2018 Nov 16;20(22):7293-7297. doi: 10.1021/acs.orglett.8b03228. Epub 2018 Nov 5. Org Lett. 2018. PMID: 30394756 Free PMC article.
-
Cytotoxic Spliceostatin Analogs from Pseudomonas sp.Chem Biodivers. 2019 Sep;16(9):e1900266. doi: 10.1002/cbdv.201900266. Epub 2019 Aug 8. Chem Biodivers. 2019. PMID: 31298476
-
Enantioselective total syntheses of FR901464 and spliceostatin A and evaluation of splicing activity of key derivatives.J Org Chem. 2014 Jun 20;79(12):5697-709. doi: 10.1021/jo500800k. Epub 2014 May 30. J Org Chem. 2014. PMID: 24873648 Free PMC article.
-
Cephalostatin analogues--synthesis and biological activity.Fortschr Chem Org Naturst. 2004;87:1-80. doi: 10.1007/978-3-7091-0581-8_1. Fortschr Chem Org Naturst. 2004. PMID: 15079895 Review.
-
Development of practical syntheses of the marine anticancer agents discodermolide and dictyostatin.Nat Prod Rep. 2008 Apr;25(2):342-75. doi: 10.1039/b705661n. Epub 2008 Feb 27. Nat Prod Rep. 2008. PMID: 18389141 Review.
Cited by
-
Interchangeable SF3B1 inhibitors interfere with pre-mRNA splicing at multiple stages.RNA. 2016 Mar;22(3):350-9. doi: 10.1261/rna.053108.115. Epub 2016 Jan 7. RNA. 2016. PMID: 26742993 Free PMC article.
-
Structural basis of intron selection by U2 snRNP in the presence of covalent inhibitors.Nat Commun. 2021 Jul 23;12(1):4491. doi: 10.1038/s41467-021-24741-1. Nat Commun. 2021. PMID: 34301950 Free PMC article.
-
Synthesis and antiproliferative activity of a tetrahydrofuran analog of FR901464.Bioorg Med Chem Lett. 2024 May 15;104:129739. doi: 10.1016/j.bmcl.2024.129739. Epub 2024 Apr 8. Bioorg Med Chem Lett. 2024. PMID: 38599298 Free PMC article.
-
Structure-Activity Relationship Study of Splicing Modulators on Hsh155/SF3B1 through Chemical Synthesis and Yeast Genetics.ACS Med Chem Lett. 2024 Nov 25;15(12):2225-2230. doi: 10.1021/acsmedchemlett.4c00510. eCollection 2024 Dec 12. ACS Med Chem Lett. 2024. PMID: 39691513 Free PMC article.
-
Syntheses of spliceostatins and thailanstatins: a review.Beilstein J Org Chem. 2020 Aug 13;16:1991-2006. doi: 10.3762/bjoc.16.166. eCollection 2020. Beilstein J Org Chem. 2020. PMID: 32831956 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

