Hydroxycinnamic Acid Degradation, a Broadly Conserved Trait, Protects Ralstonia solanacearum from Chemical Plant Defenses and Contributes to Root Colonization and Virulence
- PMID: 25423265
- PMCID: PMC4329107
- DOI: 10.1094/MPMI-09-14-0292-FI
Hydroxycinnamic Acid Degradation, a Broadly Conserved Trait, Protects Ralstonia solanacearum from Chemical Plant Defenses and Contributes to Root Colonization and Virulence
Abstract
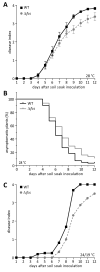
Plants produce hydroxycinnamic acid (HCA) defense compounds to combat pathogens, such as the bacterium Ralstonia solanacearum. We showed that an HCA degradation pathway is genetically and functionally conserved across diverse R. solanacearum strains. Further, a feruloyl-CoA synthetase (Δfcs) mutant that cannot degrade HCA was less virulent on tomato plants. To understand the role of HCA degradation in bacterial wilt disease, we tested the following hypotheses: HCA degradation helps the pathogen i) grow, as a carbon source; ii) spread, by reducing HCA-derived physical barriers; and iii) survive plant antimicrobial compounds. Although HCA degradation enabled R. solanacearum growth on HCA in vitro, HCA degradation was dispensable for growth in xylem sap and root exudate, suggesting that HCA are not significant carbon sources in planta. Acetyl-bromide quantification of lignin demonstrated that R. solanacearum infections did not affect the gross quantity or distribution of stem lignin. However, the Δfcs mutant was significantly more susceptible to inhibition by two HCA, namely, caffeate and p-coumarate. Finally, plant colonization assays suggested that HCA degradation facilitates early stages of infection and root colonization. Together, these results indicated that ability to degrade HCA contributes to bacterial wilt virulence by facilitating root entry and by protecting the pathogen from HCA toxicity.
Figures


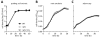

Similar articles
-
A Single Regulator Mediates Strategic Switching between Attachment/Spread and Growth/Virulence in the Plant Pathogen Ralstonia solanacearum.mBio. 2017 Sep 26;8(5):e00895-17. doi: 10.1128/mBio.00895-17. mBio. 2017. PMID: 28951474 Free PMC article.
-
The in planta transcriptome of Ralstonia solanacearum: conserved physiological and virulence strategies during bacterial wilt of tomato.mBio. 2012 Aug 31;3(4):e00114-12. doi: 10.1128/mBio.00114-12. Print 2012. mBio. 2012. PMID: 22807564 Free PMC article.
-
Ralstonia solanacearum requires PopS, an ancient AvrE-family effector, for virulence and To overcome salicylic acid-mediated defenses during tomato pathogenesis.mBio. 2013 Nov 26;4(6):e00875-13. doi: 10.1128/mBio.00875-13. mBio. 2013. PMID: 24281716 Free PMC article.
-
How Ralstonia solanacearum Exploits and Thrives in the Flowing Plant Xylem Environment.Trends Microbiol. 2018 Nov;26(11):929-942. doi: 10.1016/j.tim.2018.06.002. Epub 2018 Jun 22. Trends Microbiol. 2018. PMID: 29941188 Review.
-
Ralstonia solanacearum, a widespread bacterial plant pathogen in the post-genomic era.Mol Plant Pathol. 2013 Sep;14(7):651-62. doi: 10.1111/mpp.12038. Epub 2013 May 30. Mol Plant Pathol. 2013. PMID: 23718203 Free PMC article. Review.
Cited by
-
The Role of Hydroxycinnamic Acid Amide Pathway in Plant Immunity.Front Plant Sci. 2022 Jun 22;13:922119. doi: 10.3389/fpls.2022.922119. eCollection 2022. Front Plant Sci. 2022. PMID: 35812905 Free PMC article. Review.
-
Pseudomonas putida F1 uses energy taxis to sense hydroxycinnamic acids.Microbiology (Reading). 2017 Oct;163(10):1490-1501. doi: 10.1099/mic.0.000533. Epub 2017 Sep 28. Microbiology (Reading). 2017. PMID: 28954643 Free PMC article.
-
An MKP-MAPK protein phosphorylation cascade controls vascular immunity in plants.Sci Adv. 2022 Mar 11;8(10):eabg8723. doi: 10.1126/sciadv.abg8723. Epub 2022 Mar 9. Sci Adv. 2022. PMID: 35263144 Free PMC article.
-
Plant-like bacterial expansins play contrasting roles in two tomato vascular pathogens.Mol Plant Pathol. 2018 May;19(5):1210-1221. doi: 10.1111/mpp.12611. Epub 2017 Dec 18. Mol Plant Pathol. 2018. PMID: 28868644 Free PMC article.
-
Metabolomic Evaluation of Ralstonia solanacearum Cold Shock Protein Peptide (csp22)-Induced Responses in Solanum lycopersicum.Front Plant Sci. 2022 Jan 7;12:803104. doi: 10.3389/fpls.2021.803104. eCollection 2021. Front Plant Sci. 2022. PMID: 35069661 Free PMC article.
References
-
- Abdelkafi S, Sayadi S, Gam A, Ben Z, Casalot L, Labat M. Bioconversion of ferulic acid to vanillic acid by Halomonas elongata isolated from table-olive fermentation. FEMS Microbiol. Lett. 2006;262:115–120. - PubMed
-
- Alvarez S, Marsh EL, Schroeder SG, Schachtman DP. Metabolomic and proteomic changes in the xylem sap of maize under drought. Plant Cell Environ. 2008;31:325–340. - PubMed
-
- Balcerzak M, Harris LJ, Subramaniam R, Ouellet T. The feruloyl esterase gene family of Fusarium graminearum is differentially regulated by aromatic compounds and hosts. Fungal Biol. 2012;116:478–488. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

