Impact of a switch to fingolimod versus staying on glatiramer acetate or beta interferons on patient- and physician-reported outcomes in relapsing multiple sclerosis: post hoc analyses of the EPOC trial
- PMID: 25424122
- PMCID: PMC4253981
- DOI: 10.1186/s12883-014-0220-1
Impact of a switch to fingolimod versus staying on glatiramer acetate or beta interferons on patient- and physician-reported outcomes in relapsing multiple sclerosis: post hoc analyses of the EPOC trial
Abstract
Background: The Evaluate Patient OutComes (EPOC) study assessed physician- and patient-reported outcomes in individuals with relapsing multiple sclerosis who switched directly from injectable disease-modifying therapy (iDMT; glatiramer acetate, intramuscular or subcutaneous interferon beta-1a, or interferon beta-1b) to once-daily, oral fingolimod. Post hoc analyses evaluated the impact of a switch to fingolimod versus staying on each of the four individual iDMTs.
Methods: Overall, 1053 patients were randomized 3:1 to switch to fingolimod or remain on iDMT. The primary endpoint was the change in Treatment Satisfaction Questionnaire for Medication (TSQM) Global Satisfaction score. Secondary endpoints included changes in scores for TSQM Effectiveness, Side Effects and Convenience subscales, Beck Depression Inventory-II (BDI-II), Fatigue Severity Scale (FSS), Patient-Reported Outcome Indices for Multiple Sclerosis (PRIMUS) Activities, 36-item Short-Form Health Survey (SF-36) Mental Component Summary (MCS) and Physical Component Summary (PCS) and mean investigator-reported Clinical Global Impressions of Improvement (CGI-I). All outcomes were evaluated after 6 months of treatment.
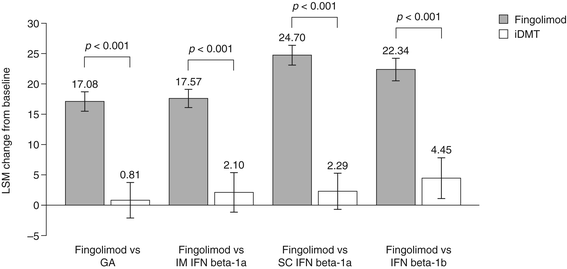
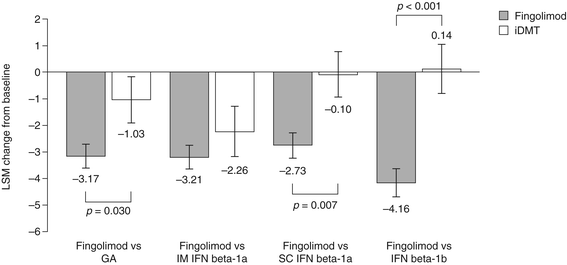
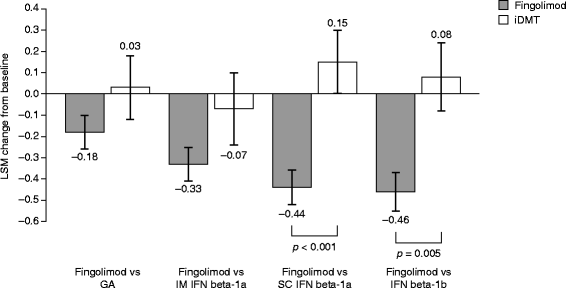
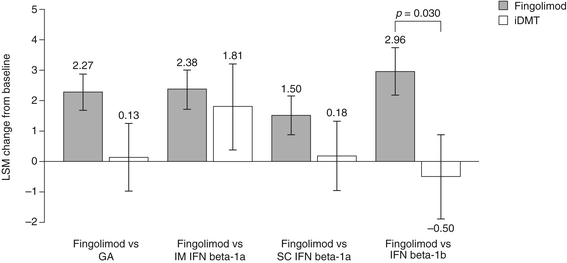
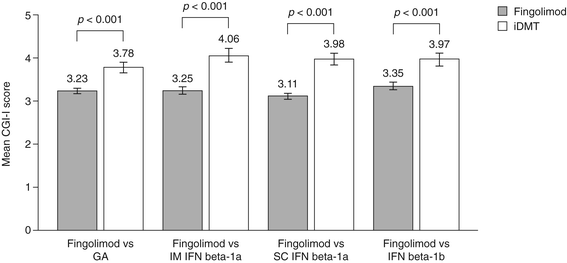
Results: Changes in TSQM Global Satisfaction scores were superior after a switch to fingolimod when compared with scores in patients remaining on any of the iDMTs (all p <0.001). Likewise, all TSQM subscale scores improved following a switch to fingolimod (all p <0.001), except when compared with glatiramer acetate for the TSQM Side Effects subscale (p = 0.111). FSS scores were found to be superior for fingolimod versus remaining on subcutaneous interferon beta-1a and interferon beta-1b, BDI-II scores were significantly improved for fingolimod except for the comparison with intramuscular interferon beta-1a, and SF-36 scores were superior with fingolimod compared with remaining on interferon beta-1b (MCS and PCS; p = 0.030 and p = 0.022, respectively) and subcutaneous interferon beta-1a (PCS only; p = 0.024). Mean CGI-I scores were superior with fingolimod when compared with continuing treatment with any of the iDMTs (all p <0.001).
Conclusions: After 6 months, a switch to fingolimod showed superiority compared with remaining on each iDMT for a range of patient- and physician-reported outcomes, including global satisfaction with treatment.
Trial registration: ClinicalTrials.gov NCT01216072 .
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

