The role of skeletal-muscle-based thermogenic mechanisms in vertebrate endothermy
- PMID: 25424279
- PMCID: PMC4854186
- DOI: 10.1111/brv.12157
The role of skeletal-muscle-based thermogenic mechanisms in vertebrate endothermy
Abstract
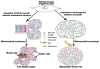
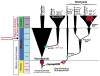
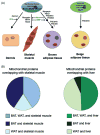
Thermogenesis is one of the most important homeostatic mechanisms that evolved during vertebrate evolution. Despite its importance for the survival of the organism, the mechanistic details behind various thermogenic processes remain incompletely understood. Although heat production from muscle has long been recognized as a thermogenic mechanism, whether muscle can produce heat independently of contraction remains controversial. Studies in birds and mammals suggest that skeletal muscle can be an important site of non-shivering thermogenesis (NST) and can be recruited during cold adaptation, although unequivocal evidence is lacking. Much research on thermogenesis during the last two decades has been focused on brown adipose tissue (BAT). These studies clearly implicate BAT as an important site of NST in mammals, in particular in newborns and rodents. However, BAT is either absent, as in birds and pigs, or is only a minor component, as in adult large mammals including humans, bringing into question the BAT-centric view of thermogenesis. This review focuses on the evolution and emergence of various thermogenic mechanisms in vertebrates from fish to man. A careful analysis of the existing data reveals that muscle was the earliest facultative thermogenic organ to emerge in vertebrates, long before the appearance of BAT in eutherian mammals. Additionally, these studies suggest that muscle-based thermogenesis is the dominant mechanism of heat production in many species including birds, marsupials, and certain mammals where BAT-mediated thermogenesis is absent or limited. We discuss the relevance of our recent findings showing that uncoupling of sarco(endo)plasmic reticulum Ca(2+)-ATPase (SERCA) by sarcolipin (SLN), resulting in futile cycling and increased heat production, could be the basis for NST in skeletal muscle. The overall goal of this review is to highlight the role of skeletal muscle as a thermogenic organ and provide a balanced view of thermogenesis in vertebrates.
Keywords: SR calcium transport; brown adipose tissue; endothermy; skeletal muscle; thermogenesis.
© 2014 Cambridge Philosophical Society.
Figures


References
-
- Anunciado-Koza RP, Zhang J, Ukropec J, Bajpeyi S, Koza RA, Rogers RC, Cefalu WT, Mynatt RL, Kozak LP. Inactivation of the mitochondrial carrier SLC25A25 (ATP-Mg2+/Pi transporter) reduces physical endurance and metabolic efficiency in mice. Journal of Biological Chemistry. 2011;286:11659–11671. - PMC - PubMed
-
- Arruda AP, Ketzer LA, Nigro M, Galina A, Carvalho DP, de Meis L. Cold tolerance in hypothyroid rabbits: role of skeletal muscle mitochondria and sarcoplasmic reticulum Ca2+ ATPase isoform 1 heat production. Endocrinology. 2008;149:6262–6271. - PubMed
-
- Arruda AP, Nigro M, Oliveira GM, de Meis L. Thermogenic activity of Ca2+ —ATPase from skeletal muscle heavy sarcoplasmic reticulum: the role of ryanodine Ca2+ channel. Biochimica et Biophysica Acta. 2007;1768:1498–1505. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

