Post-translational modifications of histones that influence nucleosome dynamics
- PMID: 25424540
- PMCID: PMC4375056
- DOI: 10.1021/cr500350x
Post-translational modifications of histones that influence nucleosome dynamics
Figures
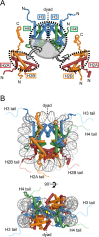
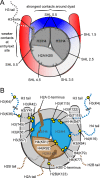

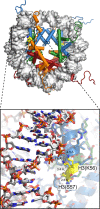
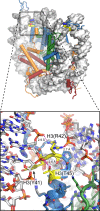

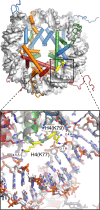
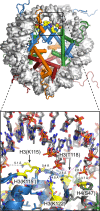
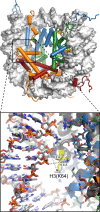
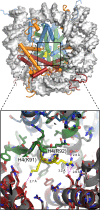

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

