Convergent differential regulation of SLIT-ROBO axon guidance genes in the brains of vocal learners
- PMID: 25424606
- PMCID: PMC4329046
- DOI: 10.1002/cne.23719
Convergent differential regulation of SLIT-ROBO axon guidance genes in the brains of vocal learners
Abstract
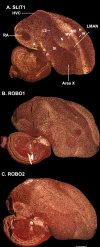
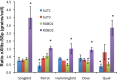
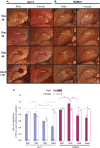
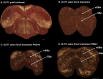
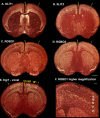
Only a few distantly related mammals and birds have the trait of complex vocal learning, which is the ability to imitate novel sounds. This ability is critical for speech acquisition and production in humans, and is attributed to specialized forebrain vocal control circuits that have several unique connections relative to adjacent brain circuits. As a result, it has been hypothesized that there could exist convergent changes in genes involved in neural connectivity of vocal learning circuits. In support of this hypothesis, expanding on our related study (Pfenning et al. [2014] Science 346: 1256846), here we show that the forebrain part of this circuit that makes a relatively rare direct connection to brainstem vocal motor neurons in independent lineages of vocal learning birds (songbird, parrot, and hummingbird) has specialized regulation of axon guidance genes from the SLIT-ROBO molecular pathway. The SLIT1 ligand was differentially downregulated in the motor song output nucleus that makes the direct projection, whereas its receptor ROBO1 was developmentally upregulated during critical periods for vocal learning. Vocal nonlearning bird species and male mice, which have much more limited vocal plasticity and associated circuits, did not show comparable specialized regulation of SLIT-ROBO genes in their nonvocal motor cortical regions. These findings are consistent with SLIT and ROBO gene dysfunctions associated with autism, dyslexia, and speech sound language disorders and suggest that convergent evolution of vocal learning was associated with convergent changes in the SLIT-ROBO axon guidance pathway.
Keywords: axon guidance; hummingbird; neural connectivity; parrot; songbird; vocal learning.
© 2014 The Authors. Wiley Periodicals, Inc.
Figures




References
-
- Bates TC, Luciano M, Medland SE, Montgomery GW, Wright MJ, Martin NG. Genetic variance in a component of the language acquisition device: ROBO1 polymorphisms associated with phonological buffer deficits. Behav Genet. 2011;41:50–57. - PubMed
-
- Blockus H, Chédotal A. The multifaceted roles of Slits and Robos in cortical circuits: from proliferation to axon guidance and neurological diseases. Curr Opin Neurobiol. 2014;27:82–88. - PubMed
-
- Bottjer SW, Brady JD, Cribbs B. Connections of a motor cortical region in zebra finches: relation to pathways for vocal learning. J Comp Neurol. 2000;420:244–260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

