Dynamic transition in supercritical iron
- PMID: 25424664
- PMCID: PMC4244626
- DOI: 10.1038/srep07194
Dynamic transition in supercritical iron
Abstract
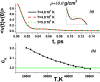
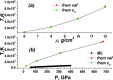
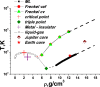
Recent advance in understanding the supercritical state posits the existence of a new line above the critical point separating two physically distinct states of matter: rigid liquid and non-rigid gas-like fluid. The location of this line, the Frenkel line, remains unknown for important real systems. Here, we map the Frenkel line on the phase diagram of supercritical iron using molecular dynamics simulations. On the basis of our data, we propose a general recipe to locate the Frenkel line for any system, the recipe that importantly does not involve system-specific detailed calculations and relies on the knowledge of the melting line only. We further discuss the relationship between the Frenkel line and the metal-insulator transition in supercritical liquid metals. Our results enable predicting the state of supercritical iron in several conditions of interest. In particular, we predict that liquid iron in the Jupiter core is in the "rigid liquid" state and is highly conducting. We finally analyse the evolution of iron conductivity in the core of smaller planets such as Earth and Venus as well as exoplanets: as planets cool off, the supercritical core undergoes the transition to the rigid-liquid conducting state at the Frenkel line.
Figures


References
-
- de Wijs G. A. et al. The viscosity of liquid iron at the physical conditions of the Earths inner core. Nature 392, 805–807 (1998).
-
- Starikov S. V. & Stegailov V. V. Premelting of iron and aluminum: implication for high-pressure melting curve measurements. Phys. Rev. B 80, 220104 (2009).
-
- Ziman J. M. Models of Disorder: The theoretical physics of homogeneously disordered systems (Cambridge Univ. Press, 1979).
-
- Likal'ter A. A. Gaseous Metals. Sov. Phys. Usp. 35, 591 (1992).
-
- Likal'ter A. A. Critical points of condensation in Coulomb systems. Phys. Usp. 43, 777 (2000).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

