Efficacy of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical intraepithelial neoplasia and cervical infection in young Japanese women
- PMID: 25424783
- PMCID: PMC4186043
- DOI: 10.4161/hv.28712
Efficacy of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical intraepithelial neoplasia and cervical infection in young Japanese women
Abstract
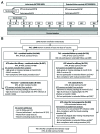
In this open, extended follow-up study (NCT00929526, Clinicaltrials.gov), we evaluated the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine efficacy, immunogenicity and safety up to 4 years after first vaccination in Japanese women aged 20-25 years. In the initial randomized, double-blind study (NCT00316693), 1040 women received the study vaccine or hepatitis A control vaccine; 752 women were included in the follow-up study. In women from the according-to-protocol efficacy cohort (ATP-E), who were initially seronegative for the HPV type analyzed, no cervical intraepithelial neoplasia (CIN) grade 1 or greater (CIN1+) cases associated with HPV-16/18 were reported in the HPV group, while in the control group, 5 cases were identified in extended follow-up analyses (vaccine efficacy [VE] 100% [95% CI: -3.7-100]) and 8 cases in combined initial and follow-up studies analyses (VE 100% [42.2-100]). In the ATP-E, VE against CIN1+ and CIN2+ associated with high-risk HPV types reached 66.4% (21.6-87.1) and 83.0% (22.1-98.2) in extended follow-up analyses, and 63.4% (28.8-82.3) and 77.3% (30.4-94.4) in analyses of combined studies, respectively. During the 4-year period, protection against CIN1+ and CIN2+, irrespective of the HPV type, was 56.7% (32.8-72.6) and 54.9% (20.5-75.3) in women receiving ≥1 vaccine dose, regardless of baseline serostatus (total vaccinated cohort [TVC]) and 61.0% (11.8-84.2) and 73.9% (1.1-95.3) in women naïve to HPV infection at baseline (TVC-naïve), respectively. The high VE observed in Japanese women, accompanied by a sustained immune response and a clinically acceptable safety profile, support findings of large, international trials.
Keywords: HPV-16/18 AS04-adjuvanted vaccine; Japan; cervical cancer; efficacy; human papillomavirus; safety.
Figures


References
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v2.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet]. Lyon, France: International Agency for Research on Cancer; 2010. Available from: http://globocan.iarc.fr [Accessed: 16 Aug. 2013].
-
- WHO/ICO Information Centre on HPV and Cervical Cancer (HPV Information Centre). Human Papillomavirus and Related Cancers in Japan. Summary Report 2010. Available at: http://www.hpvcentre.net/statistics/reports/JPN.pdf [Accessed: 16 Aug. 2013].
-
- Bosch FX, Burchell AN, Schiffman M, Giuliano AR, de Sanjose S, Bruni L, Tortolero-Luna G, Kjaer SK, Muñoz N. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;26(Suppl 10):K1–16. doi: 10.1016/j.vaccine.2008.05.064. - DOI - PubMed
-
- Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, Snijders PJ, Meijer CJ, International Agency for Research on Cancer Multicenter Cervical Cancer Study Group Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
