Laser vaccine adjuvants. History, progress, and potential
- PMID: 25424797
- PMCID: PMC4186024
- DOI: 10.4161/hv.28840
Laser vaccine adjuvants. History, progress, and potential
Abstract
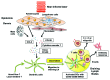
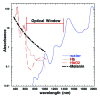
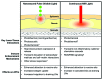
Immunologic adjuvants are essential for current vaccines to maximize their efficacy. Unfortunately, few have been found to be sufficiently effective and safe for regulatory authorities to permit their use in vaccines for humans and none have been approved for use with intradermal vaccines. The development of new adjuvants with the potential to be both efficacious and safe constitutes a significant need in modern vaccine practice. The use of non-damaging laser light represents a markedly different approach to enhancing immune responses to a vaccine antigen, particularly with intradermal vaccination. This approach, which was initially explored in Russia and further developed in the US, appears to significantly improve responses to both prophylactic and therapeutic vaccines administered to the laser-exposed tissue, particularly the skin. Although different types of lasers have been used for this purpose and the precise molecular mechanism(s) of action remain unknown, several approaches appear to modulate dendritic cell trafficking and/or activation at the irradiation site via the release of specific signaling molecules from epithelial cells. The most recent study, performed by the authors of this review, utilized a continuous wave near-infrared laser that may open the path for the development of a safe, effective, low-cost, simple-to-use laser vaccine adjuvant that could be used in lieu of conventional adjuvants, particularly with intradermal vaccines. In this review, we summarize the initial Russian studies that have given rise to this approach and comment upon recent advances in the use of non-tissue damaging lasers as novel physical adjuvants for vaccines.
Keywords: cancer; influenza; laser; near-infrared; vaccine adjuvant.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
