Acute disseminated encephalomyelitis with severe neurological outcomes following virosomal seasonal influenza vaccine
- PMID: 25424806
- PMCID: PMC4186031
- DOI: 10.4161/hv.28961
Acute disseminated encephalomyelitis with severe neurological outcomes following virosomal seasonal influenza vaccine
Abstract
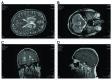
Acute disseminated encephalomyelitis (ADEM) is an inflammatory, usually monophasic, immune mediate, demyelinating disease of the central nervous system which involves the white matter. ADEM is more frequent in children and usually occurs after viral infections, but may follow vaccinations, bacterial infections, or may occur without previous events. Only 5% of cases of ADEM are preceded by vaccination within one month prior to symptoms onset. The diagnosis of ADEM requires both multifocal involvement and encephalopathy and specific demyelinating lesions of white matter. Overall prognosis of ADEM patients is often favorable, with full recovery reported in 23% to 100% of patients from pediatric cohorts, and more severe outcome in adult patients. We describe the first case of ADEM occurred few days after administration of virosomal seasonal influenza vaccine. The patient, a 59-year-old caucasic man with unremarkable past medical history presented at admission decreased alertness, 10 days after flu vaccination. During the 2 days following hospitalization, his clinical conditions deteriorated with drowsiness and fever until coma. The magnetic resonance imaging of the brain showed multiple and symmetrical white matter lesions in both cerebellar and cerebral hemispheres, suggesting demyelinating disease with inflammatory activity, compatible with ADEM. The patient was treated with high dose of steroids and intravenous immunoglobulin with relevant sequelae and severe neurological outcomes.
Keywords: ADEM; acute disseminated encephalomyelitis; adverse event; influenza vaccination; virosomal vaccine.
Figures

References
-
- World Health Organization: Fact sheet Number 211 Influenza [Internet]; updated March 2014. Available from http://www.who.int/mediacentre/factsheets/fs211.
-
- World Health Organization (WHO) Influenza vaccines. Wkly Epidemiol Rec. 2005;80:279–87. - PubMed
-
- Centers for Disease Control and Prevention (CDC) Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices–United States, 2013-2014. MMWR Recomm Rep. 2013;62:1–43. - PubMed
-
- Jefferson T, Di Pietrantonj C, Rivetti A, Bawazeer GA, Al-Ansary LA, Ferroni E. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2010;7:CD001269. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
