Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine: final analysis of a long-term follow-up study up to 9.4 years post-vaccination
- PMID: 25424918
- PMCID: PMC4896780
- DOI: 10.4161/hv.29532
Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine: final analysis of a long-term follow-up study up to 9.4 years post-vaccination
Abstract
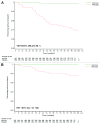
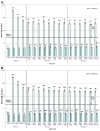
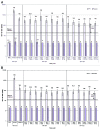
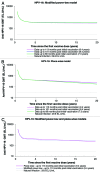
HPV-023 (NCT00518336; ClinicalTrial.gov) is a long-term follow-up of an initial double-blind, randomized (1:1), placebo-controlled study (HPV-001, NCT00689741) evaluating the efficacy against human papillomavirus (HPV)-16/18 infection and associated cyto-histopathological abnormalities, persistence of immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine. Among the women, aged 15-25 years, enrolled in HPV-001 and who participated in the follow-up study HPV-007 (NCT00120848), a subset of 437 women from five Brazilian centers participated in this 36-month long-term follow-up (HPV-023) for a total of 113 months (9.4 years). During HPV-023, anti-HPV-16/18 antibodies were measured annually by enzyme-linked immunosorbent assay (ELISA) and pseudovirion-based neutralisation assay (PBNA). Cervical samples were tested for HPV DNA every 6 months, and cyto-pathological examinations were performed annually. During HPV-023, no new HPV-16/18-associated infections and cyto-histopathological abnormalities occurred in the vaccine group. Vaccine efficacy (VE) against HPV-16/18 incident infection was 100% (95%CI: 66.1, 100). Over the 113 months (9.4 years), VE was 95.6% (86.2, 99.1; 3/50 cases in vaccine and placebo groups, respectively) against incident infection, 100% (84·1, 100; 0/21) against 6-month persistent infection (PI); 100% (61·4, 100; 0/10) against 12-month PI; 97·1% (82.5, 99.9; 1/30) against ≥ ASC-US; 95·0% (68.0, 99.9; 1/18) against ≥ LSIL; 100% (45.2, 100; 0/8) against CIN1+; and 100% (-128.1, 100; 0/3) against CIN2+ associated with HPV-16/18. All vaccinees remained seropositive to HPV-16/18, with antibody titers remaining several folds above natural infection levels, as measured by ELISA and PBNA. There were no safety concerns. To date, these data represent the longest follow-up reported for a licensed HPV vaccine.
Keywords: HPV-16/18 AS04-adjuvanted vaccine; cervical cancer; efficacy; human papillomavirus (HPV); immunogenicity; long-term protection; pre-cancer; safety.
Figures

References
-
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJF, Peto J, Meijer CJLM, Muñoz N. . Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12 - 9; http://dx.doi.org/10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH43... PMID: 10451482 - DOI - PubMed
-
- Bosch FX, Lorincz A, Muñoz N, Meijer CJLM, Shah KV. . The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 2002; 55:244 - 65; http://dx.doi.org/10.1136/jcp.55.4.244; PMID: 11919208 - DOI - PMC - PubMed
-
- Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, Snijders PJ, Meijer CJLM, International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. . Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348:518 - 27; http://dx.doi.org/10.1056/NEJMoa021641; PMID: 12571259 - DOI - PubMed
-
- Schiffman M, Clifford G, Buonaguro FM. . Classification of weakly carcinogenic human papillomavirus types: addressing the limits of epidemiology at the borderline. Infect Agent Cancer 2009; 4:8; http://dx.doi.org/10.1186/1750-9378-4-8; PMID: 19486508 - DOI - PMC - PubMed
-
- de Sanjose S, Quint WGV, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin H-R, et al. , Retrospective International Survey and HPV Time Trends Study Group. . Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11:1048 - 56; http://dx.doi.org/10.1016/S1470-2045(10)70230-8; PMID: 20952254 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
