Reversal of severe angioproliferative pulmonary arterial hypertension and right ventricular hypertrophy by combined phosphodiesterase-5 and endothelin receptor inhibition
- PMID: 25425003
- PMCID: PMC4255666
- DOI: 10.1186/s12967-014-0314-y
Reversal of severe angioproliferative pulmonary arterial hypertension and right ventricular hypertrophy by combined phosphodiesterase-5 and endothelin receptor inhibition
Abstract
Background: Patients with pulmonary arterial hypertension (PAH) are treated with vasodilators, including endothelin receptor antagonists (ERAs), phosphodiesterase-5 (PDE-5) inhibitors, soluble guanylyl cyclase activators, and prostacyclin. Despite recent advances in pharmacotherapy for individuals with PAH, morbidity and mortality rates in this patient population remain unacceptably high. Here, we tested the hypothesis that combination therapy with two PAH drugs that target distinct biochemical pathways will provide superior efficacy relative to monotherapy in the rat SU5416 plus hypoxia (SU-Hx) model of severe angioproliferative PAH, which closely mimics the human condition.
Methods: Male Sprague Dawley rats were injected with a single dose of SU5416, which is a VEGF receptor antagonist, and exposed to hypobaric hypoxia for three weeks. Rats were subsequently housed at Denver altitude and treated daily with the PDE-5 inhibitor, tadalafil (TAD), the type A endothelin receptor (ETA) antagonist, ambrisentan (AMB), or a combination of TAD and AMB for four additional weeks.
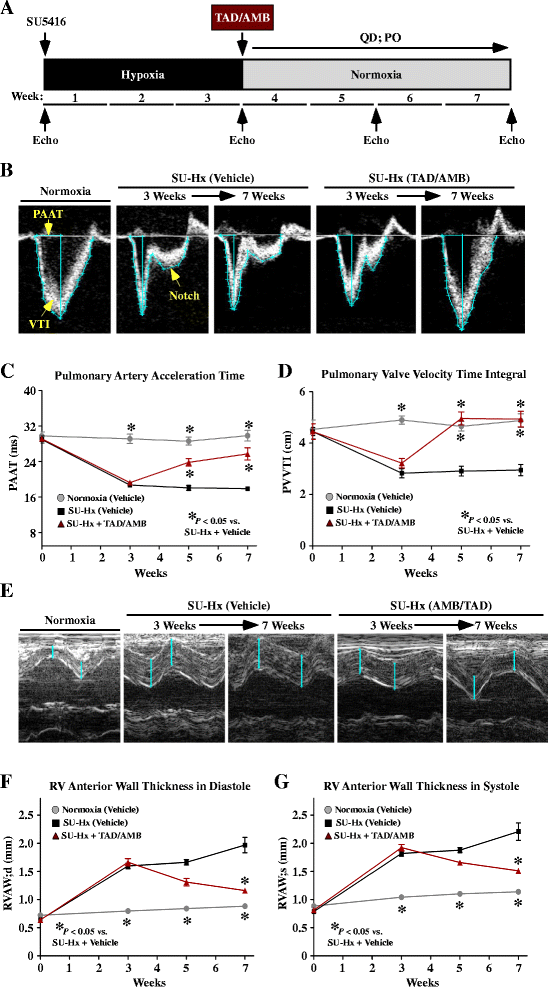
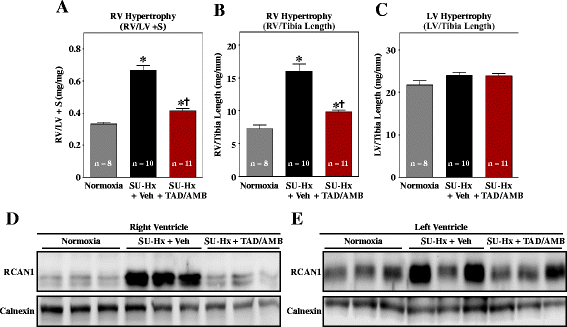
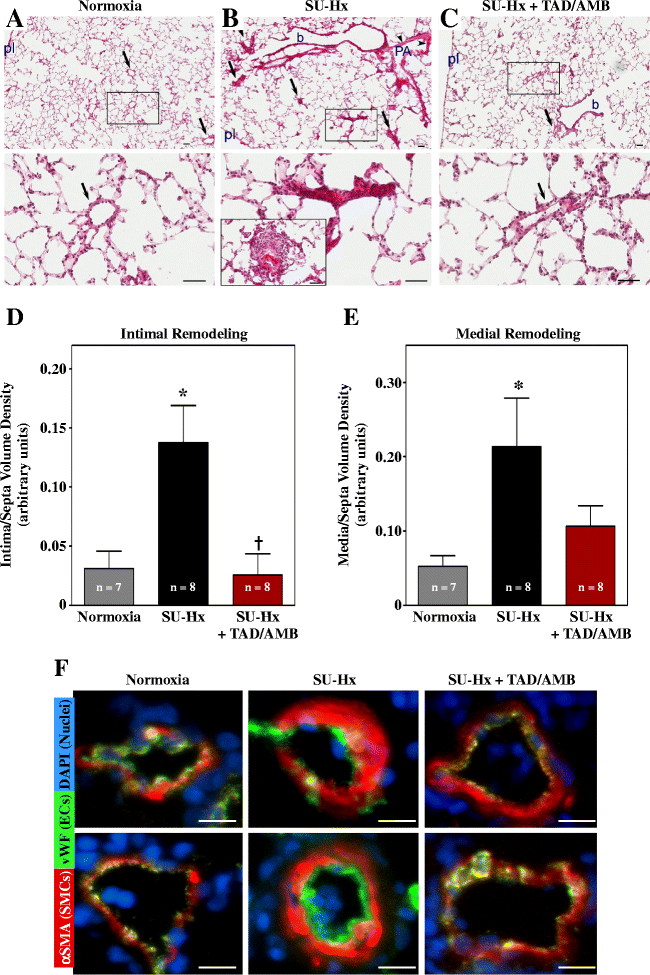
Results: Monotherapy with TAD or AMB led to modest reductions in pulmonary arterial pressure (PAP) and right ventricular (RV) hypertrophy. In contrast, echocardiography and invasive hemodynamic measurements revealed that combined TAD/AMB nearly completely reversed pulmonary hemodynamic impairment, RV hypertrophy, and RV functional deficit in SU-Hx rats. Efficacy of TAD/AMB was associated with dramatic reductions in pulmonary vascular remodeling, including suppression of endothelial cell plexiform lesions, which are common in human PAH.
Conclusions: Combined therapy with two vasodilators that are approved for the treatment of human PAH provides unprecedented efficacy in the rat SU-Hx preclinical model of severe, angioproliferative PAH.
Figures



References
-
- Hoeper MM, Barst RJ, Bourge RC, Feldman J, Frost AE, Galie N, Gomez-Sanchez MA, Grimminger F, Grunig E, Hassoun PM, Morrell NW, Peacock AJ, Satoh T, Simonneau G, Tapson VF, Torres F, Lawrence D, Quinn DA, Ghofrani HA. Imatinib mesylate as add-on therapy for pulmonary arterial hypertension: results of the randomized IMPRES study. Circulation. 2013;127:1128–1138. doi: 10.1161/CIRCULATIONAHA.112.000765. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

