Pseudomonas aeruginosa in cystic fibrosis patients with G551D-CFTR treated with ivacaftor
- PMID: 25425629
- PMCID: PMC4342673
- DOI: 10.1093/cid/ciu944
Pseudomonas aeruginosa in cystic fibrosis patients with G551D-CFTR treated with ivacaftor
Abstract
Background: Ivacaftor improves outcomes in cystic fibrosis (CF) patients with the G551D mutation; however, effects on respiratory microbiology are largely unknown. This study examines changes in CF respiratory pathogens with ivacaftor and correlates them with baseline characteristics and clinical response.
Methods: The G551D Observational Study enrolled a longitudinal observational cohort of US patients with CF aged 6 years and older with at least 1 copy of the G551D mutation. Results were linked with retrospective and prospective culture data in the US Cystic Fibrosis Foundation's National Patient Registry. Pseudomonas aeruginosa infection category in the year before and year after ivacaftor was compared and correlated with clinical findings.
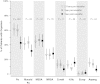
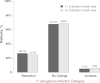
Results: Among 151 participants prescribed ivacaftor, 29% (26/89) who were culture positive for P. aeruginosa the year prior to ivacaftor use were culture negative the year following treatment; 88% (52/59) of those P. aeruginosa free remained uninfected. The odds of P. aeruginosa positivity in the year after ivacaftor compared with the year prior were reduced by 35% (odds ratio [OR], 0.65; P < .001). Ivacaftor was also associated with reduced odds of mucoid P. aeruginosa (OR, 0.77; P = .013) and Aspergillus (OR, 0.47; P = .039), but not Staphylococcus aureus or other common CF pathogens. Patients with intermittent culture positivity and higher forced expiratory volume in 1 second (FEV1) were most likely to turn culture negative. Reduction in P. aeruginosa was not associated with change in FEV1, body mass index, or hospitalizations.
Conclusions: Pseudomonas aeruginosa culture positivity was significantly reduced following ivacaftor treatment. Efficacious CFTR modulation may contribute to lower frequency of culture positivity for P. aeruginosa and other respiratory pathogens, particularly in patients with less established disease.
Keywords: CFTR modulator; P. aeruginosa; cystic fibrosis; ivacaftor.
© The Author 2014. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. - PubMed
-
- Gibbs GE. Sweat tests for diagnosis of cystic fibrosis. Q Rev Pediatr. 1958;13:188–91. - PubMed
-
- Kerem E, Corey M, Gold R, Levison H. Pulmonary function and clinical course in patients with cystic fibrosis after pulmonary colonization with Pseudomonas aeruginosa. J Pediatr. 1990;116:714–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

