Use of chimeras, point mutants, and molecular modeling to map the antagonist-binding site of 4,4',4″,4‴-(carbonylbis-(imino-5,1,3-benzenetriylbis(carbonylimino)))tetrakisbenzene-1,3-disulfonic acid (NF449) at P2X1 receptors for ATP
- PMID: 25425641
- PMCID: PMC4340402
- DOI: 10.1074/jbc.M114.592246
Use of chimeras, point mutants, and molecular modeling to map the antagonist-binding site of 4,4',4″,4‴-(carbonylbis-(imino-5,1,3-benzenetriylbis(carbonylimino)))tetrakisbenzene-1,3-disulfonic acid (NF449) at P2X1 receptors for ATP
Abstract
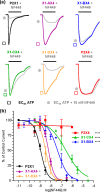
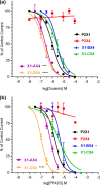
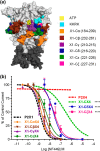
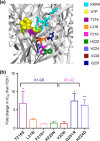
P2X receptor subtype-selective antagonists are promising candidates for treatment of a range of pathophysiological conditions. However, in contrast to high resolution structural understanding of agonist action in the receptors, comparatively little is known about the molecular basis of antagonist binding. We have generated chimeras and point mutations in the extracellular ligand-binding loop of the human P2X1 receptor, which is inhibited by NF449, suramin, and pyridoxal-phosphate-6-azophenyl-2,4-disulfonate, with residues from the rat P2X4 receptor, which is insensitive to these antagonists. There was little or no effect on sensitivity to suramin and pyridoxal-phosphate-6-azophenyl-2,4-disulfonate in chimeric P2X1/4 receptors, indicating that a significant number of residues required for binding of these antagonists are present in the P2X4 receptor. Sensitivity to the P2X1 receptor-selective antagonist NF449 was reduced by ∼60- and ∼135-fold in chimeras replacing the cysteine-rich head, and the dorsal fin region below it in the adjacent subunit, respectively. Point mutants identified the importance of four positively charged residues at the base of the cysteine-rich head and two variant residues in the dorsal fin for high affinity NF449 binding. These six residues were used as the starting area for molecular docking. The four best potential NF449-binding poses were then discriminated by correspondence with the mutagenesis data and an additional mutant to validate the binding of one lobe of NF449 within the core conserved ATP-binding pocket and the other lobes coordinated by positive charge on the cysteine-rich head region and residues in the adjacent dorsal fin.
Keywords: ATP; Electrophysiology; Ion Channel; Ligand-gated Ion Channel; Mutagenesis; NF449; P2X Receptor; PPADS; Purinergic Receptor; Suramin.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Burnstock G. (2012) Purinergic signalling: its unpopular beginning, its acceptance and its exciting future. BioEssays 34, 218–225 - PubMed
-
- Valera S., Hussy N., Evans R. J., Adami N., North R. A., Surprenant A., Buell G. (1994) A new class of ligand-gated ion channel defined by P2X receptor for extracellular ATP. Nature 371, 516–519 - PubMed
-
- Brake A. J., Wagenbach M. J., Julius D. (1994) New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature 371, 519–523 - PubMed
-
- Surprenant A., North R. A. (2009) Signaling at purinergic P2X receptors. Annu. Rev. Physiol. 71, 333–359 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

