Variomics screen identifies the re-entrant loop of the calcium-activated chloride channel ANO1 that facilitates channel activation
- PMID: 25425649
- PMCID: PMC4294516
- DOI: 10.1074/jbc.M114.618140
Variomics screen identifies the re-entrant loop of the calcium-activated chloride channel ANO1 that facilitates channel activation
Abstract
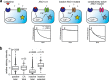
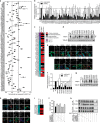
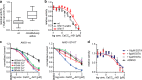
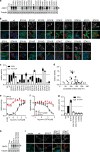
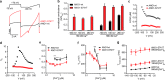
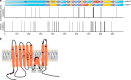
The calcium-activated chloride channel ANO1 regulates multiple physiological processes. However, little is known about the mechanism of channel gating and regulation of ANO1 activity. Using a high-throughput, random mutagenesis-based variomics screen, we generated and functionally characterized ∼6000 ANO1 mutants and identified novel mutations that affected channel activity, intracellular trafficking, or localization of ANO1. Mutations such as S741T increased ANO1 calcium sensitivity and rendered ANO1 calcium gating voltage-independent, demonstrating a critical role of the re-entrant loop in coupling calcium and voltage sensitivity of ANO1 and hence in regulating ANO1 activation. Our data present the first unbiased and comprehensive study of the structure-function relationship of ANO1. The novel ANO1 mutants reported have diverse functional characteristics, providing new tools to study ANO1 function in biological systems, paving the path for a better understanding of the function of ANO1 and its role in health and diseases.
Keywords: ANO1; Chloride Channel; Ion Channel; Mutagenesis; Protein Structure; Site-directed Mutagenesis; TMEM16A; Variomics.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Caputo A., Caci E., Ferrera L., Pedemonte N., Barsanti C., Sondo E., Pfeffer U., Ravazzolo R., Zegarra-Moran O., Galietta L. J. (2008) TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322, 590–594 - PubMed
-
- Yang Y. D., Cho H., Koo J. Y., Tak M. H., Cho Y., Shim W. S., Park S. P., Lee J., Lee B., Kim B. M., Raouf R., Shin Y. K., Oh U. (2008) TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455, 1210–1215 - PubMed
-
- Gomez-Pinilla P. J., Gibbons S. J., Bardsley M. R., Lorincz A., Pozo M. J., Pasricha P. J., Van de Rijn M., West R. B., Sarr M. G., Kendrick M. L., Cima R. R., Dozois E. J., Larson D. W., Ordog T., Farrugia G. (2009) Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G1370–G1381 - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

