Carlactone is converted to carlactonoic acid by MAX1 in Arabidopsis and its methyl ester can directly interact with AtD14 in vitro
- PMID: 25425668
- PMCID: PMC4273391
- DOI: 10.1073/pnas.1410801111
Carlactone is converted to carlactonoic acid by MAX1 in Arabidopsis and its methyl ester can directly interact with AtD14 in vitro
Abstract
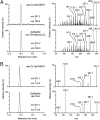
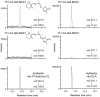
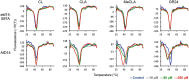
Strigolactones (SLs) stimulate seed germination of root parasitic plants and induce hyphal branching of arbuscular mycorrhizal fungi in the rhizosphere. In addition, they have been classified as a new group of plant hormones essential for shoot branching inhibition. It has been demonstrated thus far that SLs are derived from carotenoid via a biosynthetic precursor carlactone (CL), which is produced by sequential reactions of DWARF27 (D27) enzyme and two carotenoid cleavage dioxygenases CCD7 and CCD8. We previously found an extreme accumulation of CL in the more axillary growth1 (max1) mutant of Arabidopsis, which exhibits increased lateral inflorescences due to SL deficiency, indicating that CL is a probable substrate for MAX1 (CYP711A1), a cytochrome P450 monooxygenase. To elucidate the enzymatic function of MAX1 in SL biosynthesis, we incubated CL with a recombinant MAX1 protein expressed in yeast microsomes. MAX1 catalyzed consecutive oxidations at C-19 of CL to convert the C-19 methyl group into carboxylic acid, 9-desmethyl-9-carboxy-CL [designated as carlactonoic acid (CLA)]. We also identified endogenous CLA and its methyl ester [methyl carlactonoate (MeCLA)] in Arabidopsis plants using LC-MS/MS. Although an exogenous application of either CLA or MeCLA suppressed the growth of lateral inflorescences of the max1 mutant, MeCLA, but not CLA, interacted with Arabidopsis thaliana DWARF14 (AtD14) protein, a putative SL receptor, as shown by differential scanning fluorimetry and hydrolysis activity tests. These results indicate that not only known SLs but also MeCLA are biologically active in inhibiting shoot branching in Arabidopsis.
Keywords: Arabidopsis; biosynthesis; cytochrome P450; rice; strigolactone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Zwanenburg B, Pospísil T. Structure and activity of strigolactones: New plant hormones with a rich future. Mol Plant. 2013;6(1):38–62. - PubMed
-
- Akiyama K, Matsuzaki K, Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 2005;435(7043):824–827. - PubMed
-
- Booker J, et al. MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev Cell. 2005;8(3):443–449. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

