The role of drug transporters in the kidney: lessons from tenofovir
- PMID: 25426075
- PMCID: PMC4227492
- DOI: 10.3389/fphar.2014.00248
The role of drug transporters in the kidney: lessons from tenofovir
Erratum in
-
Corrigendum: The role of drug transporters in the kidney: lessons from tenofovir.Front Pharmacol. 2015 Feb 9;6:18. doi: 10.3389/fphar.2015.00018. eCollection 2015. Front Pharmacol. 2015. PMID: 25708098 Free PMC article.
Abstract
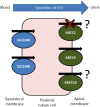
Tenofovir disoproxil fumarate, the prodrug of nucleotide reverse transcriptase inhibitor tenofovir, shows high efficacy and relatively low toxicity in HIV patients. However, long-term kidney toxicity is now acknowledged as a modest but significant risk for tenofovir-containing regimens, and continuous use of tenofovir in HIV therapy is currently under question by practitioners and researchers. Co-morbidities (hepatitis C, diabetes), low body weight, older age, concomitant administration of potentially nephrotoxic drugs, low CD4 count, and duration of therapy are all risk factors associated with tenofovir-associated tubular dysfunction. Tenofovir is predominantly eliminated via the proximal tubules of the kidney, therefore drug transporters expressed in renal proximal tubule cells are believed to influence tenofovir plasma concentration and toxicity in the kidney. We review here the current evidence that the actions, pharmacogenetics, and drug interactions of drug transporters are relevant factors for tenofovir-associated tubular dysfunction. The use of creatinine and novel biomarkers for kidney damage, and the role that drug transporters play in biomarker disposition, are discussed. The lessons learnt from investigating the role of transporters in tenofovir kidney elimination and toxicity can be utilized for future drug development and clinical management programs.
Keywords: drug transporters; kidney; pharmacokinetics; tenofovir; toxicity.
Figures
Similar articles
-
Pharmacokinetics and dosing recommendations of tenofovir disoproxil fumarate in hepatic or renal impairment.Clin Pharmacokinet. 2006;45(11):1115-24. doi: 10.2165/00003088-200645110-00005. Clin Pharmacokinet. 2006. PMID: 17048975 Clinical Trial.
-
Tenofovir renal proximal tubular toxicity is regulated by OAT1 and MRP4 transporters.Lab Invest. 2011 Jun;91(6):852-8. doi: 10.1038/labinvest.2011.48. Epub 2011 Mar 14. Lab Invest. 2011. PMID: 21403643 Free PMC article.
-
Renal Toxicity of Concomitant Exposure to Tenofovir and Inhibitors of Tenofovir's Renal Efflux Transporters in Patients Infected With HIV Type 1.J Infect Dis. 2016 Feb 15;213(4):561-8. doi: 10.1093/infdis/jiv466. Epub 2015 Sep 23. J Infect Dis. 2016. PMID: 26401025
-
Proximal tubular renal dysfunction or damage in HIV-infected patients.AIDS Rev. 2012 Jul-Sep;14(3):179-87. AIDS Rev. 2012. PMID: 22833061 Review.
-
Renal tubular transporter-mediated interactions of HIV drugs: implications for patient management.AIDS Rev. 2014 Oct-Dec;16(4):199-212. AIDS Rev. 2014. PMID: 25350530 Review.
Cited by
-
Effects of sofosbuvir-based hepatitis C treatment on the pharmacokinetics of tenofovir in HIV/HCV-coinfected individuals receiving tenofovir disoproxil fumarate.J Antimicrob Chemother. 2018 Aug 1;73(8):2112-2119. doi: 10.1093/jac/dky146. J Antimicrob Chemother. 2018. PMID: 29746648 Free PMC article. Clinical Trial.
-
Lopinavir and tenofovir interaction observed in non-pregnant adults altered during pregnancy.J Clin Pharm Ther. 2021 Oct;46(5):1459-1464. doi: 10.1111/jcpt.13477. Epub 2021 Jul 12. J Clin Pharm Ther. 2021. PMID: 34254323 Free PMC article.
-
Development and Application of Human Renal Proximal Tubule Epithelial Cells for Assessment of Compound Toxicity.Curr Chem Genom Transl Med. 2017 Feb 14;11:19-30. doi: 10.2174/2213988501711010019. eCollection 2017. Curr Chem Genom Transl Med. 2017. PMID: 28401035 Free PMC article.
-
Organic Cation Transporter 2 Overexpression May Confer an Increased Risk of Gentamicin-Induced Nephrotoxicity.Antimicrob Agents Chemother. 2016 Aug 22;60(9):5573-80. doi: 10.1128/AAC.00907-16. Print 2016 Sep. Antimicrob Agents Chemother. 2016. PMID: 27401566 Free PMC article.
-
Genetic Polymorphisms Affecting the Pharmacokinetics of Antiretroviral Drugs.Clin Pharmacokinet. 2017 Apr;56(4):355-369. doi: 10.1007/s40262-016-0456-6. Clin Pharmacokinet. 2017. PMID: 27641153 Review.
References
-
- Barditch-Crovo P., Deeks S. G., Collier A., Safrin S., Coakley D. F., Miller M., et al. (2001). Phase i/ii trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob. Agents Chemother. 45 2733–2739 10.1128/AAC.45.10.2733-2739.2001 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

