Life in the slow lane; biogeochemistry of biodegraded petroleum containing reservoirs and implications for energy recovery and carbon management
- PMID: 25426105
- PMCID: PMC4227522
- DOI: 10.3389/fmicb.2014.00566
Life in the slow lane; biogeochemistry of biodegraded petroleum containing reservoirs and implications for energy recovery and carbon management
Abstract
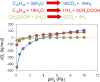
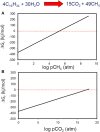
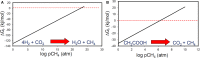
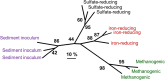
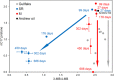
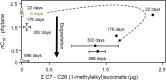
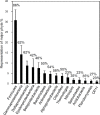
Our understanding of the processes underlying the formation of heavy oil has been transformed in the last decade. The process was once thought to be driven by oxygen delivered to deep petroleum reservoirs by meteoric water. This paradigm has been replaced by a view that the process is anaerobic and frequently associated with methanogenic hydrocarbon degradation. The thermal history of a reservoir exerts a fundamental control on the occurrence of biodegraded petroleum, and microbial activity is focused at the base of the oil column in the oil water transition zone, that represents a hotspot in the petroleum reservoir biome. Here we present a synthesis of new and existing microbiological, geochemical, and biogeochemical data that expands our view of the processes that regulate deep life in petroleum reservoir ecosystems and highlights interactions of a range of biotic and abiotic factors that determine whether petroleum is likely to be biodegraded in situ, with important consequences for oil exploration and production. Specifically we propose that the salinity of reservoir formation waters exerts a key control on the occurrence of biodegraded heavy oil reservoirs and introduce the concept of palaeopickling. We also evaluate the interaction between temperature and salinity to explain the occurrence of non-degraded oil in reservoirs where the temperature has not reached the 80-90°C required for palaeopasteurization. In addition we evaluate several hypotheses that might explain the occurrence of organisms conventionally considered to be aerobic, in nominally anoxic petroleum reservoir habitats. Finally we discuss the role of microbial processes for energy recovery as we make the transition from fossil fuel reliance, and how these fit within the broader socioeconomic landscape of energy futures.
Keywords: biogeochemistry; energy; hydrocarbon biodegradation; microbial ecology; oil reservoirs.
Figures





References
-
- Achenbach L. A., Bender K. S., Sun Y., Coates J. D. (2006). The biochemistry and genetics of microbial perchlorate reduction, in Perchlorate, Environmental Occurrence, Interactions, and Treatment, eds Gu B., Coates J. D. (New York, NY: Springer Publishers; ), 297–310.
-
- Adams J. J. (2008). The Impact of Geological and Microbiological Processes on Oil Composition and Fluid Property Variations in Heavy Oil and Bitumen Reservoirs. Ph.D. thesis, University of Calgary.
-
- Adams J. J., Jiang C., Bennett B., Huang H. P., Oldenburg T. B. P., Noke K., et al. (2008). Viscosity determination of heavy oil and bitumen. Cautions and solutions, in World Heavy Oil Conference Edmonton Paper (Calgary, AB: DMG World Media; ), 2008–443.
-
- Adams J. J., Riediger C., Fowler M., Larter S. R. (2006). Thermal controls on biodegradation around the Peace River tar sands: Paleo-pasteurization to the west. J. Geochem. Explor. 89, 1–4 10.1016/j.gexplo.2005.11.004 - DOI
-
- Adams J. J., Rostron B. J., Mendoza C. A. (2004). Coupled fluid flow, heat and mass transport, and erosion in the Alberta basin: implications for the origin of the Athabasca oil sands. Can. J. Earth Sci. 41, 1077–1095 10.1139/e04-052 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

