The progression of cell death affects the rejection of allogeneic tumors in immune-competent mice - implications for cancer therapy
- PMID: 25426116
- PMCID: PMC4227513
- DOI: 10.3389/fimmu.2014.00560
The progression of cell death affects the rejection of allogeneic tumors in immune-competent mice - implications for cancer therapy
Abstract
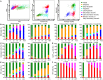
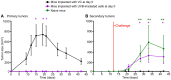
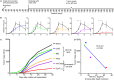
Large amounts of dead and dying cells are produced during cancer therapy and allograft rejection. Depending on the death pathway and stimuli involved, dying cells exhibit diverse features, resulting in defined physiological consequences for the host. It is not fully understood how dying and dead cells modulate the immune response of the host. To address this problem, different death stimuli were studied in B16F10 melanoma cells by regulated inducible transgene expression of the pro-apoptotic active forms of caspase-3 (revCasp-3), Bid (tBid), and the Mycobacterium tuberculosis-necrosis inducing toxin (CpnTCTD). The immune outcome elicited for each death stimulus was assessed by evaluating the allograft rejection of melanoma tumors implanted subcutaneously in BALB/c mice immunized with dying cells. Expression of all proteins efficiently killed cells in vitro (>90%) and displayed distinctive morphological and physiological features as assessed by multiparametric flow cytometry analysis. BALB/c mice immunized with allogeneic dying melanoma cells expressing revCasp-3 or CpnTCTD showed strong rejection of the allogeneic challenge. In contrast, mice immunized with cells dying either after expression of tBid or irradiation with UVB did not, suggesting an immunologically silent cell death. Surprisingly, immunogenic cell death induced by expression of revCasp-3 or CpnTCTD correlated with elevated intracellular reactive oxygen species (ROS) levels at the time point of immunization. Conversely, early mitochondrial dysfunction induced by tBid expression or UVB irradiation accounted for the absence of intracellular ROS accumulation at the time point of immunization. Although ROS inhibition in vitro was not sufficient to abrogate the immunogenicity in our allo-immunization model, we suggest that the point of ROS generation and its intracellular accumulation may be an important factor for its role as damage associated molecular pattern in the development of allogeneic responses.
Keywords: DAMPs; ROS; apoptosis; cancer; caspase-3; immunogenicity; necrosis; tBid.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

