Motion analysis and removal in intensity variation based OCT angiography
- PMID: 25426314
- PMCID: PMC4242021
- DOI: 10.1364/BOE.5.003833
Motion analysis and removal in intensity variation based OCT angiography
Abstract
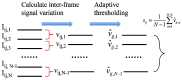
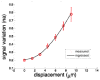
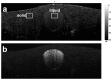
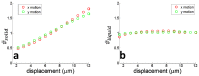
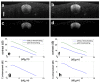
In this work, we investigated how bulk motion degraded the quality of optical coherence tomography (OCT) angiography that was obtained through calculating interframe signal variation, i.e., interframe signal variation based optical coherence angiography (isvOCA). We demonstrated theoretically and experimentally that the spatial average of isvOCA signal had an explicit functional dependency on bulk motion. Our result suggested that the bulk motion could lead to an increased background in angiography image. Based on our motion analysis, we proposed to reduce image artifact induced by transient bulk motion in isvOCA through adaptive thresholding. The motion artifact reduced angiography was demonstrated in a 1.3μm spectral domain OCT system. We implemented signal processing using graphic processing unit for real-time imaging and conducted in vivo microvasculature imaging on human skin. Our results clearly showed that the adaptive thresholding method was highly effective in the motion artifact removal for OCT angiography.
Keywords: (100.2000) Digital image processing; (110.4500) Optical coherence tomography; (170.2655) Functional monitoring and imaging.
Figures





References
-
- Zhao Y., Chen Z., Saxer C., Xiang S., de Boer J. F., Nelson J. S., “Phase-resolved optical coherence tomography and optical Doppler tomography for imaging blood flow in human skin with fast scanning speed and high velocity sensitivity,” Opt. Lett. 25(2), 114–116 (2000).10.1364/OL.25.000114 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
