Bacillus anthracis pXO1 plasmid encodes a putative membrane-bound bacteriocin
- PMID: 25426338
- PMCID: PMC4243335
- DOI: 10.7717/peerj.679
Bacillus anthracis pXO1 plasmid encodes a putative membrane-bound bacteriocin
Abstract
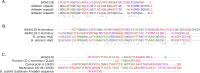
Evolutionary advantages over cousin cells in bacterial pathogens may decide about the success of a specific cell in its environment. Bacteria use a plethora of methods to defend against other cells and many devices to attack their opponents when competing for resources. Bacteriocins are antibacterial proteins that are used to eliminate competition. We report the discovery of a putative membrane-bound bacteriocin encoded by the Bacillus anthracis pathogenic pXO1 plasmid. We analyze the genomic structure of the bacteriocin operon. The proposed mechanisms of action predestine this operon as a potent competitive advantage over cohabitants of the same niche.
Keywords: BXA0140; Bacillus anthracis; Bacteriocin; Cell adherence; Cell attachment; Hemolysin II; pXO1.
Figures


References
-
- Cintas LM, Casaus P, Herranz C, Hâvarstein LS, Holo H, Hernández PE, Nes IF. Biochemical and genetic evidence that Enterococcus faecium L50 produces enterocins L50A and L50B, the sec-dependent enterocin P, and a novel bacteriocin secreted without an N-terminal extension termed enterocin Q. Journal of Bacteriology. 2000;182:6806–6814. doi: 10.1128/JB.182.23.6806-6814.2000. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

