High-fat diet-induced changes in liver thioredoxin and thioredoxin reductase as a novel feature of insulin resistance
- PMID: 25426412
- PMCID: PMC4239481
- DOI: 10.1016/j.fob.2014.10.015
High-fat diet-induced changes in liver thioredoxin and thioredoxin reductase as a novel feature of insulin resistance
Abstract
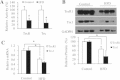
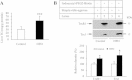
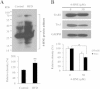
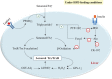
High-fat diet (HFD) can induce oxidative stress. Thioredoxin (Trx) and thioredoxin reductase (TrxR) are critical antioxidant proteins but how they are affected by HFD remains unclear. Using HFD-induced insulin-resistant mouse model, we show here that liver Trx and TrxR are significantly decreased, but, remarkably, the degree of their S-acylation is increased after consuming HFD. These HFD-induced changes in Trx/TrxR may reflect abnormalities of lipid metabolism and insulin signaling transduction. HFD-driven accumulation of 4-hydroxynonenal is another potential mechanism behind inactivation and decreased expression of Trx/TrxR. Thus, we propose HFD-induced impairment of liver Trx/TrxR as major contributor to oxidative stress and as a novel feature of insulin resistance.
Keywords: 4-HNE, 4-hydroxynonenal; ASK-1, apoptosis signal-regulating kinase-1; Gpx, glutathione peroxidase; HFD, high-fat diet; High-fat diet; IRS-1, insulin receptor substrate-1; ITT, insulin tolerance test; Insulin resistance; OGTT, oral glucose tolerance test; PTP-1B, protein-tyrosine phophatase-1B; S-acylation; Thioredoxin; Thioredoxin reductase; Trx, thioredoxin; TrxR, thioredoxin reductase.
Figures



Similar articles
-
The thioredoxin antioxidant system.Free Radic Biol Med. 2014 Jan;66:75-87. doi: 10.1016/j.freeradbiomed.2013.07.036. Epub 2013 Jul 27. Free Radic Biol Med. 2014. PMID: 23899494 Review.
-
A thioredoxin reductase and/or thioredoxin system-based mechanism for antioxidant effects of ambroxol.Biochimie. 2014 Feb;97:92-103. doi: 10.1016/j.biochi.2013.09.024. Epub 2013 Oct 5. Biochimie. 2014. PMID: 24103200
-
Inhibition of thioredoxin and thioredoxin reductase by 4-hydroxy-2-nonenal in vitro and in vivo.J Am Chem Soc. 2006 Feb 15;128(6):1879-85. doi: 10.1021/ja057358l. J Am Chem Soc. 2006. PMID: 16464088
-
Activity assays of mammalian thioredoxin and thioredoxin reductase: fluorescent disulfide substrates, mechanisms, and use with tissue samples.Anal Biochem. 2014 Mar 15;449:139-46. doi: 10.1016/j.ab.2013.12.025. Epub 2013 Dec 27. Anal Biochem. 2014. PMID: 24374250
-
Thioredoxin system in cell death progression.Antioxid Redox Signal. 2012 Dec 15;17(12):1738-47. doi: 10.1089/ars.2012.4650. Epub 2012 Jun 11. Antioxid Redox Signal. 2012. PMID: 22530689 Review.
Cited by
-
Inhibition of Src homology 2 domain-containing phosphatase 1 increases insulin sensitivity in high-fat diet-induced insulin-resistant mice.FEBS Open Bio. 2016 Jan 4;6(3):179-89. doi: 10.1002/2211-5463.12000. eCollection 2016 Mar. FEBS Open Bio. 2016. PMID: 27047746 Free PMC article.
-
The Role of Mitochondria in Sex-Dependent Differences in Hepatic Steatosis and Oxidative Stress in Response to Cafeteria Diet-Induced Obesity in Mice.Nutrients. 2019 Jul 16;11(7):1618. doi: 10.3390/nu11071618. Nutrients. 2019. PMID: 31315289 Free PMC article.
-
Extracting functionally accurate context-specific models of Atlantic salmon metabolism.NPJ Syst Biol Appl. 2023 May 27;9(1):19. doi: 10.1038/s41540-023-00280-x. NPJ Syst Biol Appl. 2023. PMID: 37244928 Free PMC article.
-
Pathways Linking Nicotinamide Adenine Dinucleotide Phosphate Production to Endoplasmic Reticulum Protein Oxidation and Stress.Front Mol Biosci. 2022 May 4;9:858142. doi: 10.3389/fmolb.2022.858142. eCollection 2022. Front Mol Biosci. 2022. PMID: 35601828 Free PMC article. Review.
-
Lipid-induced S-palmitoylation as a Vital Regulator of Cell Signaling and Disease Development.Int J Biol Sci. 2021 Oct 11;17(15):4223-4237. doi: 10.7150/ijbs.64046. eCollection 2021. Int J Biol Sci. 2021. PMID: 34803494 Free PMC article. Review.
References
-
- Vial G. Effects of a high-fat diet on energy metabolism and ROS production in rat liver. J. Hepatol. 2011;54:348–356. - PubMed
-
- Matsuzawa-Nagata N. Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metabolism. 2008;57:1071–1077. - PubMed
-
- Zhong L., Holmgren A. Essential role of selenium in the catalytic activities of mammalian thioredoxin reductase revealed by characterization of recombinant enzymes with selenocysteine mutations. J. Biol. Chem. 2000;275:18121–18128. - PubMed
-
- Bjornstedt M., Xue J., Huang W., Akesson B., Holmgren A. The thioredoxin and glutaredoxin systems are efficient electron donors to human plasma glutathione peroxidase. J. Biol. Chem. 1994;269:29382–29384. - PubMed
-
- Cha M.K., Kim I.H. Thioredoxin-linked peroxidase from human red blood cell: evidence for the existence of thioredoxin and thioredoxin reductase in human red blood cell. Biochem. Biophys. Res. Commun. 1995;217:900–907. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

