Physiological and pathological impact of blood sampling by retro-bulbar sinus puncture and facial vein phlebotomy in laboratory mice
- PMID: 25426941
- PMCID: PMC4245142
- DOI: 10.1371/journal.pone.0113225
Physiological and pathological impact of blood sampling by retro-bulbar sinus puncture and facial vein phlebotomy in laboratory mice
Abstract
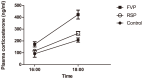
Retro-bulbar sinus puncture and facial vein phlebotomy are two widely used methods for blood sampling in laboratory mice. However, the animal welfare implications associated with these techniques are currently debated, and the possible physiological and pathological implications of blood sampling using these methods have been sparsely investigated. Therefore, this study was conducted to assess and compare the impacts of blood sampling by retro-bulbar sinus puncture and facial vein phlebotomy. Blood was obtained from either the retro-bulbar sinus or the facial vein from male C57BL/6J mice at two time points, and the samples were analyzed for plasma corticosterone. Body weights were measured at the day of blood sampling and the day after blood sampling, and the food consumption was recorded automatically during the 24 hours post-procedure. At the end of study, cheeks and orbital regions were collected for histopathological analysis to assess the degree of tissue trauma. Mice subjected to facial vein phlebotomy had significantly elevated plasma corticosterone levels at both time points in contrast to mice subjected to retro-bulbar sinus puncture, which did not. Both groups of sampled mice lost weight following blood sampling, but the body weight loss was higher in mice subjected to facial vein phlebotomy. The food consumption was not significantly different between the two groups. At gross necropsy, subcutaneous hematomas were found in both groups and the histopathological analyses revealed extensive tissue trauma after both facial vein phlebotomy and retro-bulbar sinus puncture. This study demonstrates that both blood sampling methods have a considerable impact on the animals' physiological condition, which should be considered whenever blood samples are obtained.
Conflict of interest statement
Figures





References
-
- Martini L, Lorenzini RN, Cinotti S, Fini M, Giavaresi G, et al. (2000) Evaluation of pain and stress levels of animals used in experimental research. J Surg Res 88:114–119. - PubMed
-
- van Herck H, Baumans V, De Boer SF (1994) Assessment of discomfort in laboratory animals. In: Cohen IR, Miller Aeditors. Autoimmune Disease Models. Academic Press. pp. 303–320.
-
- Tsigos C, Chrousos GP (2002) Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res 53:865–871. - PubMed
-
- Grouzmann E, Cavadas C, Grand D, Moratel M, Aubert JF, et al. (2003) Blood sampling methodology is crucial for precise measurement of plasma catecholamines concentrations in mice. Pflugers Arch - Eur J Physiol 447:254–258. - PubMed
-
- Hui Y, Huang NH, Ebbert L, Bina H, Chiang A, et al. (2007) Pharmacokinetic comparisons of tail-bleeding with cannula- or retro-orbital bleeding techniques in rats using six marketed drugs. J Pharmacol Toxicol Methods 56:256–264. - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

