Successional changes in the chicken cecal microbiome during 42 days of growth are independent of organic acid feed additives
- PMID: 25427406
- PMCID: PMC4251860
- DOI: 10.1186/s12917-014-0282-8
Successional changes in the chicken cecal microbiome during 42 days of growth are independent of organic acid feed additives
Abstract
Background: Poultry remains a major source of foodborne bacterial infections. A variety of additives with presumed anti-microbial and/or growth-promoting effects are commonly added to poultry feed during commercial grow-out, yet the effects of these additives on the gastrointestinal microbial community (the GI microbiome) as the bird matures remain largely unknown. Here we compared temporal changes in the cecal microbiome to the effects of formic acid, propionic acid, and medium-chain fatty acids (MCFA) added to feed and/or drinking water.
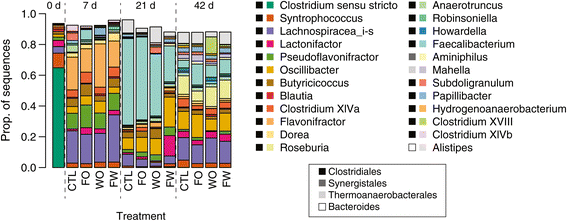
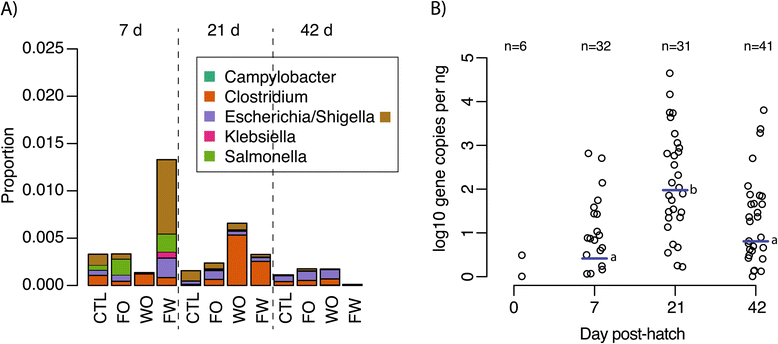
Results: Cecal bacterial communities at day of hatch (n = 5 birds), 7d (n = 32), 21d (n = 27), and 42d (n = 36) post-hatch were surveyed using direct 454 sequencing of 16S rRNA gene amplicons from each bird in combination with cultivation-based recovery of a Salmonella Typhimurium marker strain and quantitative-PCR targeting Clostridium perfringens. Treatment effects on specific pathogens were generally non-significant. S. Typhimurium introduced by oral gavage at day of hatch was recovered by cultivation from nearly all birds sampled across treatments at 7d and 21d, but by 42d, S. Typhimurium was only recovered from ca. 25% of birds, regardless of treatment. Sequencing data also revealed non-significant treatment effects on genera containing known pathogens and on the cecal microbiome as a whole. In contrast, temporal changes in the cecal microbiome were dramatic, highly significant, and consistent across treatments. At 7d, the cecal community was dominated by three genera (Flavonifractor, Pseudoflavonifractor, and a Lachnospiracea sequence type) that accounted for more than half of sequences. By 21d post-hatch, a single genus (Faecalibacterium) accounted for 23-55% of sequences, and the number of Clostridium 16S rRNA gene copies detected by quantitative-PCR reached a maximum.
Conclusions: Over the 42 d experiment, the cecal bacterial community changed significantly as measured by a variety of ecological metrics and increases in the complexity of co-occurrence networks. Management of poultry to improve animal health, nutrition, or food safety may need to consider the interactive effects of any treatments with the dramatic temporal shifts in the taxonomic composition of the cecal microbiome as described here.
Figures


References
-
- James WO, Brewer RL, Prucha JC, Williams WO, Jr, Parham DR. Effects of chlorination of chill water on the bacteriologic profile of raw chicken carcasses and giblets. J Am Vet Med Assoc. 1992;200:60–63. - PubMed
-
- James C, Vincent C, De Andrade Lima TI, James SJ. The primary chilling of poultry carcasses-a review. Int J Refrigeration. 2006;29:847–862. doi: 10.1016/j.ijrefrig.2005.08.003. - DOI
Publication types
MeSH terms
Substances
Associated data
- BioProject/263495
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

