Sulfasalazine for ankylosing spondylitis
- PMID: 25427435
- PMCID: PMC11182569
- DOI: 10.1002/14651858.CD004800.pub3
Sulfasalazine for ankylosing spondylitis
Abstract
Background: Ankylosing spondylitis (AS) is a chronic inflammatory disease of unknown cause and affects mainly the spine, but can also affect other joints. Disease progression may result in loss of mobility and function. Sulfasalazine is a disease-modifying antirheumatic drug used in the treatment of AS. However, its efficacy remains unclear. This is an update of a Cochrane review first published in 2005.
Objectives: To evaluate the benefits and harms of sulfasalazine for the treatment of ankylosing spondylitis (AS).
Search methods: We searched for relevant randomized and quasi-randomized trials in any language, using the following sources: the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 11); MEDLINE (2003 to 28 November 2013); EMBASE (2003 to 27 November 2013); CINAHL (2003 to 28 November 2013); Ovid MEDLINE data, World Health Organization International Clinical Trials Registry Platform (28 November 2013); and the reference sections of retrieved articles.
Selection criteria: We evaluated randomized and quasi-randomized trials examining the benefits and harms of sulfasalazine on AS.
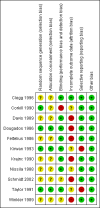
Data collection and analysis: Two review authors independently reviewed unblinded trial reports according to the selection criteria. Disagreements on the inclusion of the studies were resolved, when necessary, by recourse to a third review author. The same authors independently assessed the risk of bias of included trials and entered the data extracted from the included trials. We combined results using mean difference (MD) or standardised mean difference (SMD) for continuous data, and risk ratio (RR) for dichotomous data.We restructured outcome measures for this update based on recommendations from the editorial group. Major outcomes included: pain, Bath ankylosing spondylitis disease activity index (BASDAI), Bath ankylosing spondylitis function index (BASFI), Bath ankylosing spondylitis metrology index (BASMI), radiographic progression, total number of withdrawals due to adverse events, and serious adverse events.
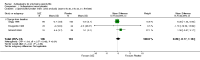
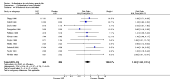
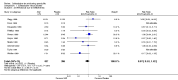
Main results: We did not add any new studies to this review following the updated search. In the original review, we included 11 studies in the analysis, involving 895 participants in total. All included studies compared sulfasalazine with placebo. We judged most of the studies as low risk of bias or unclear risk of bias in five domains (random sequence generation, allocation concealment, blinding of outcome assessment, selective reporting, and other sources of bias). However, for incomplete outcome data, we only judged one trial at low risk of bias.None of the included trials assessed BASDAI, BASFI, BASMI or radiographic progression. Different parameters were used to assess pain. The pooled MD for back pain measured on a 0 to 100 mm visual analogue scale was -2.96 (95% confidence interval (CI) -6.33 to 0.41; absolute risk difference 3%, 95% CI 1% to 6%; 6 trials). Compared to placebo, a significantly higher rate of withdrawals due to adverse effects (RR 1.50, 95% CI 1.04 to 2.15; absolute risk difference 4%, 95% CI 0.4% to 8.8%; 11 trials) was found in the sulfasalazine group. A serious adverse reaction was reported in one patient taking sulfasalazine (Peto odds ratio 7.50, 95% CI 0.15 to 378.16).
Authors' conclusions: There is not enough evidence to support any benefit of sulfasalazine in reducing pain, disease activity, radiographic progression, or improving physical function and spinal mobility in the treatment of AS. A statistically significant benefit in reducing the erythrocyte sedimentation rate and easing spinal stiffness was mentioned in the previous version. However, the effect size was very small and not clinically meaningful. More withdrawals because of side effects occurred with sulfasalazine. Further studies, with larger sample sizes, longer duration, and using validated outcome measures are needed to verify the uncertainty of sulfasalazine in AS.
Conflict of interest statement
JC ‐ none SL ‐ none CL ‐ none
Figures








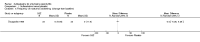
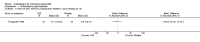



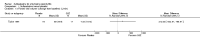






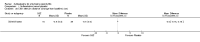








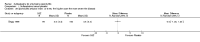
















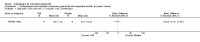



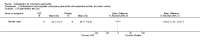
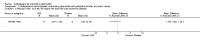


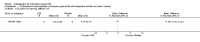
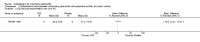
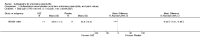

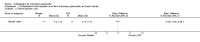
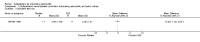
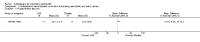


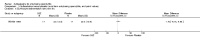
Update of
-
Sulfasalazine for ankylosing spondylitis.Cochrane Database Syst Rev. 2005 Apr 18;(2):CD004800. doi: 10.1002/14651858.CD004800.pub2. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2014 Nov 27;(11):CD004800. doi: 10.1002/14651858.CD004800.pub3. PMID: 15846731 Updated.
References
References to studies included in this review
Clegg 1996 {published data only}
-
- Clegg DO, Reda DJ, Adbellaitf M. Comparison of sulfasalazine and placebo for the treatment of axial and peripheral articular manifestations of the seronegative spondyloarthropathies: a Department of Veterans Affairs cooperative study. Arthritis and Rheumatism 1999;42(11):2325‐9. - PubMed
-
- Clegg DO, Reda DJ, Weisman MH, Blackburn WD, Cush JJ, Cannon GW, et al. Comparison of sulfasalazine and placebo in the treatment of ankylosing spondylitis: a Department of Veterans Affairs Cooperative Group. Arthritis and Rheumatism 1996;39:2004‐12. - PubMed
-
- Reda D, Anderson R, Abdellatif M, Williams D, Clegg D. Longitudinal analysis of binary data in the V.A. Cooperative Study of sulfasalazine for the treatment of seronegative spondyloarthropathies. Controlled Clinical Trials 1995;16(3 Suppl):90s‐91s.
Corkill 1990 {published data only}
-
- Corkill MM, Jobanputra P, Gibson T, Macfarlane DG. A controlled trial of sulphasalazine treatment of chronic ankylosing spondylitis: failure to demonstrate a clinical effect. British Journal of Rheumatology 1990;29(1):41‐5. - PubMed
Davis 1989 {published data only}
-
- Davis MJ, Dawes PT, Beswick E, Lewin IV, Stanworth DR. Sulphasalazine therapy in ankylosing spondylitis: its effect on disease activity, immunoglobulin A and the complex immunoglobulin A‐alpha‐1‐antitrypsin. British Journal of Rheumatology 1989;28(5):410‐3. - PubMed
Dougados 1986 {published data only}
-
- Dougados M, Boumier P, Amor B. Treatment of ankylosing spondylarthritis with salazosulfapyridin. A double‐blind, controlled study in 60 patients [French]. Revue du Rhumatisme et des Maladies Osteo Articulaires 1987;54(3):255‐60. - PubMed
-
- Dougados M, Nguyen M, Mijiyawa M, Amor B. Sulfasalazine in Spondylarthropathies. Zeitschrift fur Rheumatologie 1990;49(Suppl 1):80.
Feltelius 1986 {published data only}
Kirwan 1993 {published data only}
-
- Kirwan J, Edwards A, Huitfeldt B, Thompson P, Currey H. The course of established ankylosing spondylitis and the effects of sulphasalazine over 3 years. British Journal of Rheumatology 1993;32(8):729‐33. - PubMed
Krajnc 1990 {published data only}
-
- Krajnc I. Sulphasalazine in the treatment of ankylosing spondylitis [Serbocroatian]. Lijecnicki Vjesnik 1990;112(5‐6):171‐4. - PubMed
Nissila 1988 {published data only}
-
- Nissila M, Lahesmaa R, Leirisalo‐Repo M, Lehtinen K, Toivanen P, Granfors K. Antibodies to Klebsiella pneumoniae, Escherichia coli, and Proteus mirabilis in ankylosing spondylitis: effect of sulfasalazine treatment. Journal of Rheumatology 1994;21(11):2082‐7. - PubMed
-
- Nissila M, Lehtinen K, Leirisalo‐Repo M, Luukkainen R, Mutru O, Yli‐Kerttula U. Sulfasalazine in the treatment of ankylosing spondylitis. A twenty‐six‐week, placebo‐controlled clinical trial. Arthritis and Rheumatism 1988;31(9):1111‐6. - PubMed
Schmidt 2002 {published data only}
-
- Schmidt WA, Wierth S, Milleck D, Droste U, Gromnica‐Ihle E. Sulfasalazine in ankylosing spondylitis: a prospective, randomized, double‐blind placebo‐controlled study and comparison with other controlled studies [German]. Zeitschrift fur Rheumatologie 2002;61(2):159‐67. - PubMed
-
- Schmidt WA, Wierth S, Milleck D, Droste U, Gromnica‐Ihle E. Sulphasalazine in ankylosing spondylitis: a prospective, randomized, double‐blind, placebo‐controlled study and meta‐analysis of other controlled studies. Zeitschrift fur Rheumatologie 2009;59(Suppl 3):69. - PubMed
Taylor 1991 {published data only}
-
- Taylor HG, Beswick EJ, Dawes PT. Sulphasalazine in ankylosing spondylitis. A radiological, clinical and laboratory assessment. Clinical Rheumatology 1991;10(1):43‐8. - PubMed
Winkler 1989 {published data only}
-
- Winkler V. Sulphasalazine treatment in ankylosing spondylitis: A comparison of sulphasalazine with placebo. Magyar Reumatologia 1989;30(Suppl):29‐37.
References to studies excluded from this review
Benitez‐Del‐Castillo {published data only}
-
- Benitez‐Del‐Castillo JM, Garcia‐Sanchez J, Iradier T, Banares A. Sulfasalazine in the prevention of anterior uveitis associated with ankylosing spondylitis. Eye 2000;14(3A):340‐3. - PubMed
Braun 2006 {published data only}
-
- Braun J, Zochling J, Baraliakos X, Alten R, Burmester G, Grasedyck K, et al. Efficacy of sulfasalazine in patients with inflammatory back pain due to undifferentiated spondyloarthritis and early ankylosing spondylitis: a multicentre randomised controlled trial. Annals of the Rheumatic Diseases 2006;65(9):1147‐53. - PMC - PubMed
Braun 2011 {published data only}
-
- Braun J, Pavelka K, Ramos‐Remus C, Dimic A, Vlahos B, Freundlich B, et al. Clinical efficacy of etanercept versus sulfasalazine in ankylosing spondylitis subjects with peripheral joint involvement. Journal of Rheumatology 2012;39(4):836‐40. - PubMed
-
- Braun J, Horst‐Bruinsma IE, Huang F, Burgos‐Vargas R, Vlahos B, Koenig AS, et al. Clinical efficacy and safety of etanercept versus sulfasalazine in patients with ankylosing spondylitis: A randomized, double‐blind trial. Arthritis and Rheumatism 2011;63(6):1543‐51. - PubMed
-
- Freundlich B, Braun J, Huang F, Burgos‐Vargas R, Horst‐Bruinsma IE, Vlahos B, et al. Assessment of clinical efficacy in a randomized, double‐blind study of etanercept and sulphasalazine in patients with ankylosing spondylitis. Internal Medicine Journal 2009;39:A55.
-
- Guzman R, Burgos‐Vargas R, Braun J, Freundlich B, Vlahos B, Koenig AS. Etanercept is significantly more effective than sulfasalazine in patients with ankylosing spondylitis. Journal of Clinical Rheumatology 2010;16:S73.
-
- NCT00247962. Study Evaluating Etanercept and Sulphasalazine in Ankylosing Spondylitis. clinicaltrials.gov/ct2/show/record/NCT00247962 2008.
Burgos‐Vargas 2002 {published data only}
-
- Burgos‐Vargas R, Pacheco‐Tena C, Vazquez‐Mellado J. A short‐term follow‐up of enthesitis and arthritis in the active phase of juvenile onset spondyloarthropathies. Clinical and Experimental Rheumatology 2002;20(5):727‐31. - PubMed
Deng 2009 {published data only}
-
- Deng X, Huang F, Zhang J. Thalidomide delays the rate of relapse in ankylosing spondylitis after discontinuing etanercept treatment [停用依那西普后应用沙立度胺可延缓强直性脊柱炎的复发]. Arthritis and Rheumatism 2009;60:1776.
NCT00953979 {published data only}
-
- NCT00953979. Efficacy and safety of Kunxian capsule in treatment of patients with early ankylosing spondylitis [昆仙胶囊治疗强直性脊柱炎的疗效及安全性]. clinicaltrials.gov/ct2/show/record/NCT00953979.
Song 2011 {published data only}
-
- Song I‐H, Althoff CE, Haibel H, Hermann K‐GA, Poddubnyy D, Listing J, et al. Frequency and duration of drug‐free remission after 1 year of treatment with etanercept versus sulfasalazine in early axial spondyloarthritis: 2 year data of the ESTHER trial. Annals of the Rheumatic Diseases 2012;71(7):1212‐5. - PubMed
-
- Song IH, Hermann KG, Haibel H, Althoff CE, Listing J, Burmester GR, et al. Effects of etanercept versus sulfasalazine in early axial spondyloarthritis on active inflammatory lesions as detected by whole‐body MRI (ESTHER): A 48‐week randomised controlled trial. Annals of the Rheumatic Diseases 2011;70(4):590‐6. - PMC - PubMed
Xu 2008 {published data only}
-
- Xu JR, Zhang CY, Li WM. Effects of bushen tongdu decoction on serum tumour necrosis factor‐alpha and transforming growth factor beta1, in patients with ankylosing spondylitis [补肾通督方对强直性脊柱炎血清TNF‐α及TGF‐β_1水平的影响]. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi Jiehe Zazhi/Chinese Journal of Integrated Traditional and Western Medicine 2008;28(12):1093‐5. - PubMed
Zhao 2006 {published data only}
-
- Zhao FT, Zhao H, Guan JL, Han XH. Clinical study on long‐term effectiveness of leflunomide compared with sulfasalazine in treatment of ankylosing spondylitis [Chinese]. Pharmaceutical Care and Research 2006;6(6):430‐2.
Zhao 2009 {published data only}
-
- Zhao FT, Zhao F, Wang YL. Efficacy of etanercept on ankylosing spondylitis [依那西普治疗强直性脊柱炎的疗效分析]. Journal of Shanghai Jiaotong University (Medical Science) 2009;29(12):1506‐8.
Additional references
Akkoc 2005
-
- Akkoc N, Khan MA. Overestimation of the prevalence of ankylosing spondylitis in the Berlin study: comment on the article by Braun et al. Arthritis and Rheumatism 2005;52(12):4048‐9. - PubMed
Braun 2002
-
- Braun J, Brandt J, Listing J, Zink A, Alten R, Golder W, et al. Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicenter trial. Lancet 2002;359(9313):1187‐93. - PubMed
Braun 2011a
Chen 2013
Dagfinrud 2008
Davis 2005
-
- Davis JC, Heijde D, Dougados M, Woolley JM. Reductions in health‐related quality of life in patients with ankylosing spondylitis and improvements with etanercept therapy. Arthritis and Rheumatism 2005;53(4):494‐501. - PubMed
Feldtkeller 2003
-
- Feldtkeller E, Khan MA, Heijde D, Linden S, Braun J. Age at disease onset and diagnosis delay in HLA‐B27 negative vs. positive patients with ankylosing spondylitis. Rheumatology International 2003;23(2):61‐6. - PubMed
Ferraz 1990
-
- Ferraz MB, Tugwell P, Goldsmith CH, Atra E. Meta‐analysis of sulfasalazine in ankylosing spondylitis. The Journal of Rheumatology 1990;17(11):1482‐6. - PubMed
Gorman 2002
-
- Gorman JD, Sack KE, Davis JC. Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor alpha. New England Journal of Medicine 2002;346(18):1349‐56. - PubMed
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Olivieri 2002
-
- Olivieri I, Tubergen A, Salvarani C, Linden S. Seronegative spondyloarthritides. Best Practice and Research Clinical Rheumatology 2002;16(5):723‐39. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Smedegård 1995
-
- Smedegård G, Björk J. Sulphasalazine: mechanism of action in rheumatoid arthritis. British Journal of Rheumatology 1995;34 Suppl 2:7‐15. - PubMed
Suarez‐Almazor 1998
-
- Suarez‐Almazor ME, Belseck E, Shea B, Tugwell P, Wells GA. Sulfasalazine for treating rheumatoid arthritis. Cochrane Database of Systematic Reviews 1998, Issue 2. [DOI: 10.1002/14651858.CD000958] - DOI
van den Bosch 2002
-
- Bosch F, Kruithof E, Baeten D, Herssens A, keyser F, Mielants H, et al. Randomized double‐blind comparison of chimeric monoclonal antibody to tumor necrosis factor alpha (infliximab) versus placebo in active spondylarthropathy. Arthritis and Rheumatism 2002;46(3):755‐65. - PubMed
van der Heijde 1999
-
- Heijde D, Linden S, Bellamy N, Calin A, Dougados M, Khan MA. Which domains should be included in a core set for endpoints in ankylosing spondylitis? Introduction to the ankylosing spondylitis module of OMERACT IV. The Journal of Rheumatology 1999;26(4):945‐7. - PubMed
van der Heijde 2002
van der Heijde 2005
-
- Heijde D, Dougados M, Davis J, Weisman MH, Maksymowych W, Braun J, et al. Assessment in Ankylosing Spondylitis International Working Group/Spondylitis Association of America recommendations for conducting clinical trials in ankylosing spondylitis. Arthritis and Rheumatism 2005;52(2):386‐94. - PubMed
van der Heijde 2011
-
- Heijde D, Sieper J, Maksymowych WP, Dougados M, Burgos‐Vargas R, Landewé R, et al. Assessment of SpondyloArthritis International Society. 2010 Update of the international ASAS recommendations for the use of anti‐TNF agents in patients with axial spondyloarthritis. Annals of the Rheumatic Diseases 2011;70(6):905. - PubMed
Zink 2000
-
- Zink A, Braun J, Listing J, Wollenhaupt J. Disability and handicapin in rheumatoid arthritis and ankylosing spondylitis ‐ results from the German rheumatological database. German Collaborative Arthritis Centers. Journal of Rheumatology 2007;27(3):613‐22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

