Review of the literature and individual patients' data meta-analysis on efficacy and tolerance of nitroxoline in the treatment of uncomplicated urinary tract infections
- PMID: 25427651
- PMCID: PMC4262220
- DOI: 10.1186/s12879-014-0628-7
Review of the literature and individual patients' data meta-analysis on efficacy and tolerance of nitroxoline in the treatment of uncomplicated urinary tract infections
Abstract
Background: Nitroxoline, a hydroxychinoline derivate, has been used for many years to treat urinary tract infections (UTI). Many uncontrolled, but only few controlled clinical studies have been published. Four so far unpublished, controlled clinical studies were meta-analysed.
Methods: A narrative literature review was performed. In addition the individual patient data (IPD) of 466 females with uncomplicated UTI of four prospective, single blind, randomized, clinical studies with similar protocols using nitroxoline (250 mg tid) versus cotrimoxazole (960 mg bid) or norfloxacin (400 mg bid) as controls for 5 days (sporadic UTI) or 10 days (recurrent UTI) were meta-analysed. The primary aim was eradication of bacteriuria 7-13 days after end of therapy (test of cure). Clinical efficacy was determined by elimination of symptoms and safety by adverse events and laboratory tests.
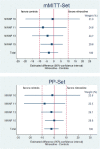
Results: Reviewing a total of 26 uncontrolled, 2 controlled and one postmarketing studies including more than 11,000 patients, good efficacy and safety of nitroxoline could be confirmed. In the four unpublished controlled studies a total of 234 patients were treated orally with nitroxoline and 232 with controls. The safety of nitroxoline was very good and comparable to the controls (adverse events 9.4% vs 7.8%; p = 0.360). In the mMITT set (at least one outcome result), in the PP set (test of cure outcome) and in the modified PP set (missing test of cure rated failure) more than 90% of the patients showed eradication of bacteriuria with nitroxoline, which also met statistical non-inferiority compared to the controls (10% non-inferiority margin) in all three evaluation sets. The clinical efficacy was similar between the two treatment groups.
Conclusion: The IPD meta-analysis using objective parameters (elimination of bacteriuria) demonstrated equivalent efficacy (non-inferiority) of nitroxoline with the controls tested (cotrimoxazole, norfloxacin) in the treatment of uncomplicated UTI. Considering the good safety and efficacy of nitroxoline as also shown in many uncontrolled and observational studies and the world wide increase of resistance of uropathogens against cotrimoxazole and fluoroquinolones, but not against nitroxoline within the last 20 years, nitroxoline should be reconsidered as one of the first line antibiotics for the treatment of uncomplicated UTI.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

