Blood pressure lowering efficacy of partial agonist beta blocker monotherapy for primary hypertension
- PMID: 25427719
- PMCID: PMC6486122
- DOI: 10.1002/14651858.CD007450.pub2
Blood pressure lowering efficacy of partial agonist beta blocker monotherapy for primary hypertension
Abstract
Background: Partial agonists are a subclass of beta blockers used to treat hypertension in many countries. Partial agonist act by stimulating beta receptors when they are quiescent and blocking beta receptors when they are active. The blood pressure (BP) lowering effect of partial agonist beta blockers has not been quantified.
Objectives: To quantify the dose-related effects of various partial agonists beta blockers on systolic blood pressure (SBP), diastolic blood pressure (DBP) and heart rate versus placebo in patients with primary hypertension.
Search methods: We searched the Hypertension Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE In-Process, EMBASE and ClinicalTrials.gov for randomized controlled trials up to October 2014. The WHO International Clinical Trials Registry Platform (ICTRP) is searched for inclusion in the Group's Specialised Register.
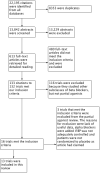
Selection criteria: Randomized double-blinded placebo-controlled parallel or cross-over trials. Studies must contain a partial agonist monotherapy arm with fixed dose. Patients enrolled into the studies must have primary hypertension at baseline (defined as SBP/DBP > 140/90 mmHg). Duration of studies must be between three to 12 weeks.
Data collection and analysis: Two authors (GW and HB) confirmed the inclusion of studies and extracted the data independently.
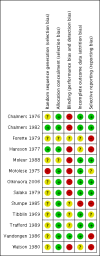
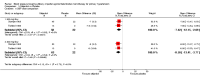
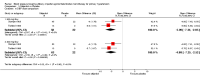
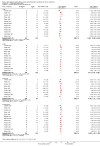
Main results: Thirteen randomized double-blinded placebo-controlled trials that examined the blood pressure lowering efficacy of six partial agonists in 605 hypertensive patients were included in this review. Five of the included studies were parallel studies and the other eight were cross-over studies. The overall risk of bias is high in this review due to the small sample size and high risk of detection bias. Pindolol, celiprolol and alprenolol lowered SBP and DBP compared to placebo. Acebutolol lowered SBP but there was no clear evidence that it lowered DBP. There was no clear evidence that pindolol and oxprenolol lowered SBP or DBP. Other than for celiprolol, sample sizes were generally small increasing the uncertainty in findings for individual agents versus placebo. In patients with moderate to severe hypertension, partial agonists (considered as a subclass) lowered peak BP by an average of 8 mmHg systolic (95% CI, -10 to -6, very low quality evidence), 4 mmHg diastolic (95%CI, -5 to -3, very low quality evidence) and reduced heart rate by five beats per minute (95%CI, -6 to -4, very low quality evidence). Higher dose partial agonists did not appear to provide additional BP lowering effects compared to lower dose. The maximum BP lowering effect of the overall subclass occurred at the starting dose. Partial agonists reduced pulse pressure by 4 mmHg (95% CI, -5 to -2, very low evidence). Only one study reported withdrawal due to adverse effects, the risk ratio (95% confidence interval) was 0.72 (0.07, 7.67).
Authors' conclusions: There was very low quality evidence that in patients with moderate to severe hypertension, partial agonists lowered peak BP by an average of 8/4 mmHg and reduced heart rate by five beats per minute. There was no evidence of a greater effect at doses higher than the initial doses. This estimate was probably exaggerated as it was subject to a high risk of bias. Based on the indirect comparison of the results in this review and two Cochrane reviews on angiotensin-converting-enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), which also used similar inclusion criteria as this review, the BP lowering effect appeared to be less than the effect in patients with mild to moderate elevated BP who were taking ACE inhibitors and ARBs based on an indirect comparison. Withdrawals due to adverse effects were only reported in one trial so it is impossible to assess the harm of these drugs.
Conflict of interest statement
James Wright: nothing to declare.
Gavin Wong: nothing to declare.
Heidi Boyda: nothing to declare.
Figures





























Update of
References
References to studies included in this review
Chalmers 1976 {published data only}
-
- Chalmer JP, Korner PI, Tiller DJ, Bune AJ, Steiner JD, West MJ, et al. Double blind factorial trial of pindolol and hydrochlorothiazide in hypertension. Medical Journal of Australia 1976;1:650‐3. [BAC‐Pindolol‐511] - PubMed
Chalmers 1982 {published data only}
-
- Chalmers JP, Wing LMH, Grygiel JJ, West MJ, Graham JR, Bune AJ. Effect of once daily indapamide and pindolol on blood pressure, plasma aldosterone concentration and plasma renin activity in a general practice setting. European Journal of Clinical Pharmacology 1982;22:191‐6. [BAC‐Pindolol‐272] - PubMed
Forette 1979 {published data only}
-
- Forette MF, Henry JF, Hervy MP, Forette B, Berthaux P. The treatment of hypertension in elderly using a beta‐blocker: acebutolol. [Traitement de l'hyertension artérielle du sujet âgé par un bêta‐bloquant: l'acébutolol]. Nouvelle Presse Médicale 1979;8:2881‐4. [BAC‐Acebutolol‐291] - PubMed
Hansson 1977 {published data only}
-
- Hansson L, Berglund G, Andersson O, Holm M. Controlled trial of acebutolol in hypertension. European Journal of Clinical Pharmacology 1977;12:89‐92. [BAP‐Acebutolol‐305] - PubMed
Moleur 1988 {published data only}
-
- Moleur P, Peyrieux JC, Luciani J, David D, Boissel JP. Bopindolol in the treatment of moderate hypertension: a dose response study. Fundamental & Clinical Pharmacology 1988;2:431‐40. [BAP‐bopindolol‐205] - PubMed
Motolese 1975 {published data only}
-
- Motolese M, Muisan G, Colombi A. Hypotensive effect of oxprenolol in mild to moderate hypertension: a multicentre controlled study. European Journal of Clinical Pharmacology 1975;8:21‐31. [BAP‐Oxprenolol‐320] - PubMed
Olkinuora 2006 {published data only}
-
- Olkinuora JT, Wiikari J, Vanhanen H, Niina M, Kalliomaki T. Effect of celiprolol and simvastatin on the calculated risk of coronary heart disease. Scandinavian Cardiovascular Journal 2006;40:160‐6. [BAP‐Celiprolol‐037] - PubMed
Salako 1979 {published data only}
-
- Salako LA, Falase AO, Fadeke Aderounmu A. Placebo‐controlled, double blind clinical trial of alprenolol in Afraican hypertensive patients. Current Medical Research and Opinion 1979;6:358‐63. [BAC‐alprenolol‐285] - PubMed
Stumpe 1985 {published data only}
-
- Stump K, Kolloch R, Mathieu M, Capone P. A comparison of celiprolol and atenolol in the treatment of hypertension: A placebo controlled double blind study. British Journal of Clinical Practice 1985;Spp 40:73‐5. [BAP‐Celiprolol‐241] - PubMed
Tibblin 1969 {published data only}
-
- Tibblin G, Ablad B. Antihypertensive therapy with alprenolol, a beta‐adrenergic receptor antagonist. Acta Medica Scandinavica 1969;186:451‐7. [BAC‐alprenolol‐352] - PubMed
Trafford 1989 {published data only}
-
- Trafford JAP, Latta D, Little PS, Parsley J, Ankier SI, Quail D. A multi‐centre, placebo controlled comparative study between 200 mg and 400 mg celiprolol in patients with mild to moderate essential hypertension. Current Medical Research and Opinion 1989;11:550‐6. [BAC‐Celiprolol‐190] - PubMed
Vandongen 1986 {published data only}
References to studies excluded from this review
Erley 1997 {published data only}
-
- Erley CM, Berger ED, Heyne N, Klass M, Kramer D, Braun N, et al. A double‐blind placebo‐controlled comparison of the effects of beta receptor blockers and ACE inhibitor on renal haemodynamics and proteinuria in chronic glomerulonephritis. Deutsche Medizinische Wochenschrift 1997;122:953‐8. - PubMed
Fraser 1986 {published data only}
-
- Fraser DM, Nimmo GR, Poloniecki JD. Acebutolol in the treatment of diabetic patients with hypertension. Current Medical Research and Opinion 1986;10:122‐7. [BAP‐Acebutolol‐232] - PubMed
Additional references
Chen 2010
eCPS
-
- Canadian Pharmacist Association. https://www.e‐therapeutics.ca/cps.showMonograph.action Accessd on OCT 2012.
EMC
-
- European Electronic Medical Compendium. http://www.medicines.org.uk/emc/default.aspx Date accessed MAY 2013.
FDA
-
- FDA Online Label Repository. http://labels.fda.gov/ Accessed on MAY 2013.
Heran 2008 (ACEI)
Heran 2008 (ARB)
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jadad 1996
-
- Jadad AR, Moore RA, Carrol D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. - PubMed
Law 2005
-
- Law M, Morris JK, Jordan R, Wald N. Headaches and the treatment of blood pressure results from a meta‐analysis of 94 randomized placebo‐controlled trials with 24 000 participants. Circulation 2005;112:2301‐6. - PubMed
Magee 2003
Wiysonge 2007
Wong 2008 (alpha and beta blockers)
Wong 2008 (beta‐1 blockers)
Wong 2014
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

