The Toll/NF-κB signaling pathway is required for epidermal wound repair in Drosophila
- PMID: 25427801
- PMCID: PMC4273363
- DOI: 10.1073/pnas.1408224111
The Toll/NF-κB signaling pathway is required for epidermal wound repair in Drosophila
Abstract
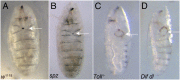
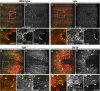
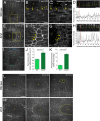
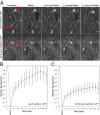
The Toll/NF-κB pathway, first identified in studies of dorsal-ventral polarity in the early Drosophila embryo, is well known for its role in the innate immune response. Here, we reveal that the Toll/NF-κB pathway is essential for wound closure in late Drosophila embryos. Toll mutants and Dif dorsal (NF-κB) double mutants are unable to repair epidermal gaps. Dorsal is activated on wounding, and Dif and Dorsal are required for the sustained down-regulation of E-cadherin, an obligatory component of the adherens junctions (AJs), at the wound edge. This remodeling of the AJs promotes the assembly of an actin-myosin cable at the wound margin; contraction of the actin cable, in turn, closes the wound. In the absence of Toll or Dif and dorsal (dl), both E-cadherin down-regulation and actin-cable formation fail, thus resulting in open epidermal gaps. Given the conservation of the Toll/NF-κB pathway in mammals and the epithelial expression of many components of the pathway, this function in wound healing is likely to be conserved in vertebrates.
Keywords: Drosophila; E-cadherin; NF-κB transcription factors; Toll pathway; epithelial wound repair.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Martin P, Lewis J. Actin cables and epidermal movement in embryonic wound healing. Nature. 1992;360(6400):179–183. - PubMed
-
- Garcia-Fernandez B, Campos I, Geiger J, Santos AC, Jacinto A. Epithelial resealing. Int J Dev Biol. 2009;53(8–10):1549–1556. - PubMed
-
- Schäfer M, Werner S. Transcriptional control of wound repair. Annu Rev Cell Dev Biol. 2007;23:69–92. - PubMed
-
- Mace KA, Pearson JC, McGinnis W. An epidermal barrier wound repair pathway in Drosophila is mediated by grainy head. Science. 2005;308(5720):381–385. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

