Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens
- PMID: 25428507
- PMCID: PMC4279952
- DOI: 10.1038/nature13988
Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens
Abstract
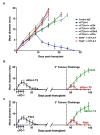
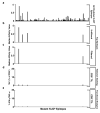
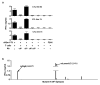
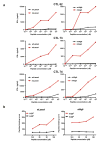
The immune system influences the fate of developing cancers by not only functioning as a tumour promoter that facilitates cellular transformation, promotes tumour growth and sculpts tumour cell immunogenicity, but also as an extrinsic tumour suppressor that either destroys developing tumours or restrains their expansion. Yet, clinically apparent cancers still arise in immunocompetent individuals in part as a consequence of cancer-induced immunosuppression. In many individuals, immunosuppression is mediated by cytotoxic T-lymphocyte associated antigen-4 (CTLA-4) and programmed death-1 (PD-1), two immunomodulatory receptors expressed on T cells. Monoclonal-antibody-based therapies targeting CTLA-4 and/or PD-1 (checkpoint blockade) have yielded significant clinical benefits-including durable responses--to patients with different malignancies. However, little is known about the identity of the tumour antigens that function as the targets of T cells activated by checkpoint blockade immunotherapy and whether these antigens can be used to generate vaccines that are highly tumour-specific. Here we use genomics and bioinformatics approaches to identify tumour-specific mutant proteins as a major class of T-cell rejection antigens following anti-PD-1 and/or anti-CTLA-4 therapy of mice bearing progressively growing sarcomas, and we show that therapeutic synthetic long-peptide vaccines incorporating these mutant epitopes induce tumour rejection comparably to checkpoint blockade immunotherapy. Although mutant tumour-antigen-specific T cells are present in progressively growing tumours, they are reactivated following treatment with anti-PD-1 and/or anti-CTLA-4 and display some overlapping but mostly treatment-specific transcriptional profiles, rendering them capable of mediating tumour rejection. These results reveal that tumour-specific mutant antigens are not only important targets of checkpoint blockade therapy, but they can also be used to develop personalized cancer-specific vaccines and to probe the mechanistic underpinnings of different checkpoint blockade treatments.
Figures
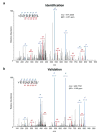
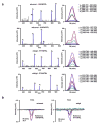
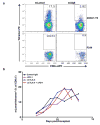
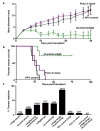
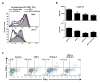

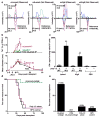
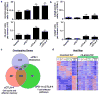
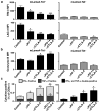
Comment in
-
Immunotherapy: personalizing tumour vaccines.Nat Rev Clin Oncol. 2015 Feb;12(2):64. doi: 10.1038/nrclinonc.2014.220. Epub 2014 Dec 16. Nat Rev Clin Oncol. 2015. PMID: 25511189 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- R01 CA190700/CA/NCI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- U01 CA141541/CA/NCI NIH HHS/United States
- P50 CA101942/CA/NCI NIH HHS/United States
- R37 CA043059/CA/NCI NIH HHS/United States
- T32 CA009547/CA/NCI NIH HHS/United States
- P30 AR048335/AR/NIAMS NIH HHS/United States
- P01 AI054456/AI/NIAID NIH HHS/United States
- P30 CA091842/CA/NCI NIH HHS/United States
- R01 AI091965/AI/NIAID NIH HHS/United States
- R01 CA043059/CA/NCI NIH HHS/United States
- T32 CA00954729/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

