Influenza A virus uses intercellular connections to spread to neighboring cells
- PMID: 25428869
- PMCID: PMC4300760
- DOI: 10.1128/JVI.03306-14
Influenza A virus uses intercellular connections to spread to neighboring cells
Abstract
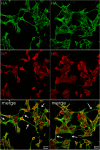
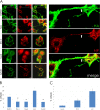
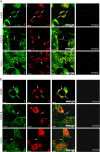
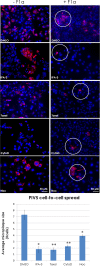
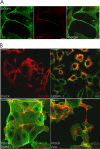
In the extracellular environment, cell-free virions seek out naive host cells over long distances and between organisms. This is the primary mechanism of spread for most viruses. Here we provide evidence for an alternative pathway previously undescribed for orthomyxoviruses, whereby the spread of influenza A virus (IAV) infectious cores to neighboring cells can occur within intercellular connections. The formation of these connections requires actin dynamics and is enhanced by viral infection. Connected cells have contiguous membranes, and the core infectious viral machinery (RNP and polymerase) was present inside the intercellular connections. A live-cell movie of green fluorescent protein (GFP)-tagged NS1 of IAV shows viral protein moving from one cell to another through an intercellular connection. The movement of tagged protein was saltatory but overall traveled only in one direction. Infectious virus cores can move from one cell to another without budding and release of cell-free virions, as evidenced by the finding that whereas a neuraminidase inhibitor alone did not inhibit the development of IAV microplaques, the presence of a neuraminidase inhibitor together with drugs inhibiting actin dynamics or the microtubule stabilizer paclitaxel (originally named taxol) precluded microplaque formation. Similar results were also observed with parainfluenza virus 5 (PIV5), a paramyxovirus, when neutralizing antibody was used to block spread by cell-free virions. Intercellular spread of infectious core particles was unaffected or enhanced in the presence of nocodazole for IAV but inhibited for PIV5. The intercellular connections have a core of filamentous actin, which hints toward transport of virus particles through the use of a myosin motor.
Importance: Here we describe a new method by which influenza A virus (IAV) spreads from cell to cell: IAV uses intracellular connections. The formation of these connections requires actin dynamics and is enhanced by viral infection and the absence of microtubules. Connected cells appeared to have contiguous membranes, and the core infectious viral machinery (RNP and polymerase) was present inside the intercellular connections. Infectious virus cores can move from one cell to another without budding and release of cell-free virions. Similar results were also observed with parainfluenza virus 5 (PIV5).
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures





References
-
- Shaw ML, Palese P. 2013. Orthomyxoviridae, p 1151–1185 Fields virology, 6th ed Lippincott Williams & Wilkins, Philadelphia, PA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

