Nonmuscle myosin heavy chain IIb mediates herpes simplex virus 1 entry
- PMID: 25428876
- PMCID: PMC4300746
- DOI: 10.1128/JVI.03079-14
Nonmuscle myosin heavy chain IIb mediates herpes simplex virus 1 entry
Abstract
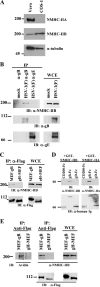
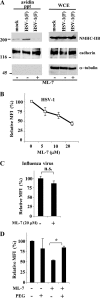
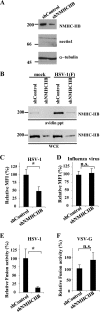
Nonmuscle myosin heavy chain IIA (NMHC-IIA) has been reported to function as a herpes simplex virus 1 (HSV-1) entry coreceptor by interacting with viral envelope glycoprotein B (gB). Vertebrates have three genetically distinct isoforms of the NMHC-II, designated NMHC-IIA, NMHC-IIB, and NMHC-IIC. COS cells, which are readily infected by HSV-1, do not express NMHC-IIA but do express NMHC-IIB. This observation prompted us to investigate whether NMHC-IIB might associate with HSV-1 gB and be involved in an HSV-1 entry like NMHC-IIA. In these studies, we show that (i) NMHC-IIB coprecipitated with gB in COS-1 cells upon HSV-1 entry; (ii) a specific inhibitor of myosin light chain kinase inhibited cell surface expression of NMHC-IIB in COS-1 cells upon HSV-1 entry as well as HSV-1 infection, as reported with NMHC-IIA; (iii) overexpression of mouse NMHC-IIB in IC21 cells significantly increased their susceptibility to HSV-1 infection; and (iv) knockdown of NMHC-IIB in COS-1 cells inhibited HSV-1 infection as well as cell-cell fusion mediated by HSV-1 envelope glycoproteins. These results supported the hypothesis that, like NMHC-IIA, NMHC-IIB associated with HSV-1 gB and mediated HSV-1 entry.
Importance: Herpes simplex virus 1 (HSV-1) was reported to utilize nonmuscle myosin heavy chain IIA (NMHC-IIA) as an entry coreceptor associating with gB. Vertebrates have three genetically distinct isoforms of NMHC-II. In these isoforms, NMHC-IIB is of special interest since it highly expresses in neuronal tissue, one of the most important cellular targets of HSV-1 in vivo. In this study, we demonstrated that the ability to mediate HSV-1 entry appeared to be conserved in NMHC-II isoforms. These results may provide an insight into the mechanism by which HSV-1 infects a wide variety of cell types in vivo.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Roizman B, Knipe DM, Whitley RJ. 2007. Herpes simplex viruses, p 2501–2601 InKnipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Strauss SE (ed), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

