A brief elevation of serum amyloid A is sufficient to increase atherosclerosis
- PMID: 25429103
- PMCID: PMC4306683
- DOI: 10.1194/jlr.M054015
A brief elevation of serum amyloid A is sufficient to increase atherosclerosis
Abstract
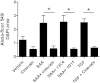
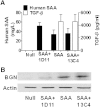
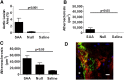
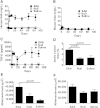
Serum amyloid A (SAA) has a number of proatherogenic effects including induction of vascular proteoglycans. Chronically elevated SAA was recently shown to increase atherosclerosis in mice. The purpose of this study was to determine whether a brief increase in SAA similarly increased atherosclerosis in a murine model. The recombination activating gene 1-deficient (rag1(-/-)) × apolipoprotein E-deficient (apoe(-/-)) and apoe(-/-) male mice were injected, multiple times or just once respectively, with an adenoviral vector encoding human SAA1 (ad-SAA); the injected mice and controls were maintained on chow for 12-16 weeks. Mice receiving multiple injections of ad-SAA, in which SAA elevation was sustained, had increased atherosclerosis compared with controls. Strikingly, mice receiving only a single injection of ad-SAA, in which SAA was only briefly elevated, also had increased atherosclerosis compared with controls. Using in vitro studies, we demonstrate that SAA treatment leads to increased LDL retention, and that prevention of transforming growth factor beta (TGF-β) signaling prevents SAA-induced increases in LDL retention and SAA-induced increases in vascular biglycan content. We propose that SAA increases atherosclerosis development via induction of TGF-β, increased vascular biglycan content, and increased LDL retention. These data suggest that even short-term inflammation with concomitant increase in SAA may increase the risk of developing CVD.
Keywords: apolipoproteins; cardiovascular disease; extracellular matrix; free-form: biglycan; lipoproteins; proteoglycans; transforming growth factor beta; vascular biology.
Figures

Similar articles
-
Serum amyloid A, but not C-reactive protein, stimulates vascular proteoglycan synthesis in a pro-atherogenic manner.Am J Pathol. 2008 Dec;173(6):1902-10. doi: 10.2353/ajpath.2008.080201. Epub 2008 Oct 30. Am J Pathol. 2008. PMID: 18974302 Free PMC article.
-
Signaling of Serum Amyloid A Through Receptor for Advanced Glycation End Products as a Possible Mechanism for Uremia-Related Atherosclerosis.Arterioscler Thromb Vasc Biol. 2016 May;36(5):800-9. doi: 10.1161/ATVBAHA.115.306349. Epub 2016 Mar 17. Arterioscler Thromb Vasc Biol. 2016. PMID: 26988587
-
Serum amyloid A and lipoprotein retention in murine models of atherosclerosis.Arterioscler Thromb Vasc Biol. 2005 Apr;25(4):785-90. doi: 10.1161/01.ATV.0000158383.65277.2b. Epub 2005 Feb 3. Arterioscler Thromb Vasc Biol. 2005. PMID: 15692094
-
Serum amyloid A in atherosclerosis.Curr Opin Lipidol. 2011 Aug;22(4):302-7. doi: 10.1097/MOL.0b013e3283488c39. Curr Opin Lipidol. 2011. PMID: 21734573 Review.
-
Serum amyloid A and atherosclerosis.Curr Opin Lipidol. 2016 Oct;27(5):531-5. doi: 10.1097/MOL.0000000000000331. Curr Opin Lipidol. 2016. PMID: 27579547 Review.
Cited by
-
High-Density Lipoproteins and Serum Amyloid A (SAA).Curr Atheroscler Rep. 2021 Jan 15;23(2):7. doi: 10.1007/s11883-020-00901-4. Curr Atheroscler Rep. 2021. PMID: 33447953 Free PMC article. Review.
-
Inflammatory remodeling of the HDL proteome impairs cholesterol efflux capacity.J Lipid Res. 2015 Aug;56(8):1519-30. doi: 10.1194/jlr.M059089. Epub 2015 May 20. J Lipid Res. 2015. PMID: 25995210 Free PMC article.
-
Serum Amyloid A is not obligatory for high-fat, high-sucrose, cholesterol-fed diet-induced obesity and its metabolic and inflammatory complications.PLoS One. 2022 Apr 18;17(4):e0266688. doi: 10.1371/journal.pone.0266688. eCollection 2022. PLoS One. 2022. PMID: 35436297 Free PMC article.
-
Inflammatory Mediators in Atherosclerotic Vascular Remodeling.Front Cardiovasc Med. 2022 May 4;9:868934. doi: 10.3389/fcvm.2022.868934. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35600479 Free PMC article. Review.
-
Serum Amyloid A Stimulates Vascular and Renal Dysfunction in Apolipoprotein E-Deficient Mice Fed a Normal Chow Diet.Front Immunol. 2019 Mar 7;10:380. doi: 10.3389/fimmu.2019.00380. eCollection 2019. Front Immunol. 2019. PMID: 30899260 Free PMC article.
References
-
- Uhlar C. M., Burgess C. J., Sharp P. M., Whitehead A. S. 1994. Evolution of the serum amyloid A (SAA) protein superfamily. Genomics. 19: 228–235. - PubMed
-
- Coetzee G. A., Strachan A. F., van der Westhuyzen D. R., Hoppe H. C., Jeenah M. S., de Beer F. C. 1986. Serum amyloid A-containing human high density lipoprotein 3. Density, size, and apolipoprotein composition. J. Biol. Chem. 261: 9644–9651. - PubMed
-
- Leinonen E., Hurt-Camejo E., Wiklund O., Hulten L. M., Hiukka A., Taskinen M. R. 2003. Insulin resistance and adiposity correlate with acute-phase reaction and soluble cell adhesion molecules in type 2 diabetes. Atherosclerosis. 166: 387–394. - PubMed
-
- O’Brien K. D., Brehm B. J., Seeley R. J., Bean J., Wener M. H., Daniels S., D’Alessio D. A. 2005. Diet-induced weight loss is associated with decreases in plasma serum amyloid A and C-reactive protein independent of dietary macronutrient composition in obese subjects. J. Clin. Endocrinol. Metab. 90: 2244–2249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

