Intrinsic neurophysiological properties of hilar ectopic and normotopic dentate granule cells in human temporal lobe epilepsy and a rat model
- PMID: 25429123
- PMCID: PMC4329435
- DOI: 10.1152/jn.00835.2014
Intrinsic neurophysiological properties of hilar ectopic and normotopic dentate granule cells in human temporal lobe epilepsy and a rat model
Abstract
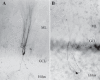
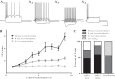
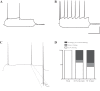
Hilar ectopic dentate granule cells (DGCs) are a salient feature of aberrant plasticity in human temporal lobe epilepsy (TLE) and most rodent models of the disease. Recent evidence from rodent TLE models suggests that hilar ectopic DGCs contribute to hyperexcitability within the epileptic hippocampal network. Here we investigate the intrinsic excitability of DGCs from humans with TLE and the rat pilocarpine TLE model with the objective of comparing the neurophysiology of hilar ectopic DGCs to their normotopic counterparts in the granule cell layer (GCL). We recorded from 36 GCL and 7 hilar DGCs from human TLE tissue. Compared with GCL DGCs, hilar DGCs in patient tissue exhibited lower action potential (AP) firing rates, more depolarized AP threshold, and differed in single AP waveform, consistent with an overall decrease in excitability. To evaluate the intrinsic neurophysiology of hilar ectopic DGCs, we made recordings from retrovirus-birthdated, adult-born DGCs 2-4 mo after pilocarpine-induced status epilepticus or sham treatment in rats. Hilar DGCs from epileptic rats exhibited higher AP firing rates than normotopic DGCs from epileptic or control animals. They also displayed more depolarized resting membrane potential and wider AP waveforms, indicating an overall increase in excitability. The contrasting findings between disease and disease model may reflect differences between the late-stage disease tissue available from human surgical specimens and the earlier disease stage examined in the rat TLE model. These data represent the first neurophysiological characterization of ectopic DGCs from human hippocampus and prospectively birthdated ectopic DGCs in a rodent TLE model.
Keywords: dentate gyrus; ectopic; epileptogenesis; intrinsic excitability; seizure.
Copyright © 2015 the American Physiological Society.
Figures

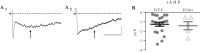




References
-
- Agrawal N, Alonso A, Ragsdale DS. Increased persistent sodium currents in rat entorhinal cortex layer V neurons in a post-status epilepticus model of temporal lobe epilepsy. Epilepsia 44: 1601–1604, 2003. - PubMed
-
- Althaus AL, Parent JM. Role of adult neurogenesis in seizure-induced hippocampal remodeling and epilepsy. In: Endogenous Stem Cell-Based Brain Remodeling in Mammals, edited by Junier M.-P., Kernie SG. Boston, MA: Springer, 2014, p. 87–104.
-
- Aradi I, Holmes WR. Role of multiple calcium and calcium-dependent conductances in regulation of hippocampal dentate granule cell excitability. J Comput Neurosci 6: 215–235, 1999. - PubMed
-
- Chen S, Su H, Yue C, Remy S, Royeck M Sochivko D, Opitz T, Beck H, Yaari Y. An increase in persistent sodium current contributes to intrinsic neuronal bursting after status epilepticus. J Neurophysiol 105: 117–129, 2011. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

