PMP22 is critical for actin-mediated cellular functions and for establishing lipid rafts
- PMID: 25429154
- PMCID: PMC4244477
- DOI: 10.1523/JNEUROSCI.1908-14.2014
PMP22 is critical for actin-mediated cellular functions and for establishing lipid rafts
Abstract
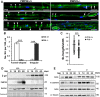
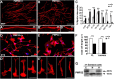
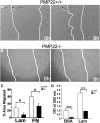
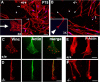
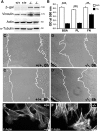
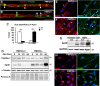
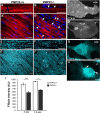
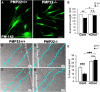
Haploinsufficiency of peripheral myelin protein 22 (PMP22) causes hereditary neuropathy with liability to pressure palsies, a peripheral nerve lesion induced by minimal trauma or compression. As PMP22 is localized to cholesterol-enriched membrane domains that are closely linked with the underlying actin network, we asked whether the myelin instability associated with PMP22 deficiency could be mediated by involvement of the protein in actin-dependent cellular functions and/or lipid raft integrity. In peripheral nerves and cells from mice with PMP22 deletion, we assessed the organization of filamentous actin (F-actin), and actin-dependent cellular functions. Using in vitro models, we discovered that, in the absence of PMP22, the migration and adhesion capacity of Schwann cells and fibroblasts are similarly impaired. Furthermore, PMP22-deficient Schwann cells produce shortened myelin internodes, and display compressed axial cell length and collapsed lamellipodia. During early postnatal development, F-actin-enriched Schmidt-Lanterman incisures do not form properly in nerves from PMP22(-/-) mice, and the expression and localization of molecules associated with uncompacted myelin domains and lipid rafts, including flotillin-1, cholesterol, and GM1 ganglioside, are altered. In addition, we identified changes in the levels and distribution of cholesterol and ApoE when PMP22 is absent. Significantly, cholesterol supplementation of the culture medium corrects the elongation and migration deficits of PMP22(-/-) Schwann cells, suggesting that the observed functional impairments are directly linked with cholesterol deficiency of the plasma membrane. Our findings support a novel role for PMP22 in the linkage of the actin cytoskeleton with the plasma membrane, likely through regulating the cholesterol content of lipid rafts.
Keywords: Schwann cell; actin cytoskeleton; cholesterol; lipid rafts; myelin; neuropathy.
Copyright © 2014 the authors 0270-6474/14/3416140-13$15.00/0.
Figures
Similar articles
-
PMP22 Regulates Cholesterol Trafficking and ABCA1-Mediated Cholesterol Efflux.J Neurosci. 2019 Jul 3;39(27):5404-5418. doi: 10.1523/JNEUROSCI.2942-18.2019. Epub 2019 May 6. J Neurosci. 2019. PMID: 31061090 Free PMC article.
-
Localization and functional roles of PMP22 in peripheral nerves of P0-deficient mice.Glia. 1999 Dec;28(3):256-64. Glia. 1999. PMID: 10559784
-
Developmental abnormalities in the nerves of peripheral myelin protein 22-deficient mice.J Neurosci Res. 2007 Feb 1;85(2):238-49. doi: 10.1002/jnr.21118. J Neurosci Res. 2007. PMID: 17131416
-
Many facets of the peripheral myelin protein PMP22 in myelination and disease.Microsc Res Tech. 1998 Jun 1;41(5):359-71. doi: 10.1002/(SICI)1097-0029(19980601)41:5<359::AID-JEMT3>3.0.CO;2-L. Microsc Res Tech. 1998. PMID: 9672419 Review.
-
Regulation of myelin-specific gene expression. Relevance to CMT1.Ann N Y Acad Sci. 1999 Sep 14;883:91-108. Ann N Y Acad Sci. 1999. PMID: 10586235 Review.
Cited by
-
Downregulation of the human peripheral myelin protein 22 gene by miR-29a in cellular models of Charcot-Marie-Tooth disease.Gene Ther. 2019 Dec;26(12):455-464. doi: 10.1038/s41434-019-0098-z. Epub 2019 Aug 27. Gene Ther. 2019. PMID: 31455873 Free PMC article.
-
Peripheral myelin protein 22 modulates store-operated calcium channel activity, providing insights into Charcot-Marie-Tooth disease etiology.J Biol Chem. 2019 Aug 9;294(32):12054-12065. doi: 10.1074/jbc.RA118.006248. Epub 2019 Jun 18. J Biol Chem. 2019. PMID: 31213528 Free PMC article.
-
Loss of YAP in Schwann cells improves HNPP pathophysiology.Glia. 2024 Nov;72(11):1974-1984. doi: 10.1002/glia.24592. Epub 2024 Jul 11. Glia. 2024. PMID: 38989661
-
Self-assembling peptide hydrogels functionalized with LN- and BDNF- mimicking epitopes synergistically enhance peripheral nerve regeneration.Theranostics. 2020 Jul 9;10(18):8227-8249. doi: 10.7150/thno.44276. eCollection 2020. Theranostics. 2020. PMID: 32724468 Free PMC article.
-
The Actin Cytoskeleton in Myelinating Cells.Neurochem Res. 2020 Mar;45(3):684-693. doi: 10.1007/s11064-019-02753-0. Epub 2019 Mar 7. Neurochem Res. 2020. PMID: 30847860 Free PMC article. Review.
References
-
- Abramoff MD, Magalhães PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11:36.
-
- Amici SA, Dunn WA, Jr, Murphy AJ, Adams NC, Gale NW, Valenzuela DM, Yancopoulos GD, Notterpek L. Peripheral myelin protein 22 is in complex with α6β4 integrin, and its absence alters the Schwann cell basal lamina. J Neurosci. 2006;26:1179–1189. doi: 10.1523/JNEUROSCI.2618-05.2006. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
