Caspase-responsive smart gadolinium-based contrast agent for magnetic resonance imaging of drug-induced apoptosis
- PMID: 25429349
- PMCID: PMC4241271
- DOI: 10.1039/C4SC01392A
Caspase-responsive smart gadolinium-based contrast agent for magnetic resonance imaging of drug-induced apoptosis
Abstract
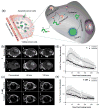
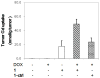
Non-invasive detection of caspase-3/7 activity in vivo has provided invaluable predictive information regarding tumor therapeutic efficacy and anti-tumor drug selection. Although a number of caspase-3/7 targeted fluorescence and positron emission tomography (PET) imaging probes have been developed, there is still a lack of gadolinium (Gd)-based magnetic resonance imaging (MRI) probes that enable high spatial resolution detection of caspase-3/7 activity in vivo. Here we employ a self-assembly approach and develop a caspase-3/7 activatable Gd-based MRI probe for monitoring tumor apoptosis in mice. Upon reduction and caspase-3/7 activation, the caspase-sensitive nano-aggregation MR probe (C-SNAM: 1) undergoes biocompatible intramolecular cyclization and subsequent self-assembly into Gd-nanoparticles (GdNPs). This results in enhanced r1 relaxivity-19.0 (post-activation) vs. 10.2 mM-1 s-1 (pre-activation) at 1 T in solution-and prolonged accumulation in chemotherapy-induced apoptotic cells and tumors that express active caspase-3/7. We demonstrate that C-SNAM reports caspase-3/7 activity by generating a significantly brighter T1-weighted MR signal compared to non-treated tumors following intravenous administration of C-SNAM, providing great potential for high-resolution imaging of tumor apoptosis in vivo.
Figures

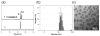


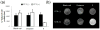
Similar articles
-
Caspase-3-Triggered Intracellular Gadolinium Nanoparticle Formation for T1-Weighted Magnetic Resonance Imaging of Apoptosis In Vivo.Nano Lett. 2023 Jul 12;23(13):6178-6183. doi: 10.1021/acs.nanolett.3c01787. Epub 2023 Jun 26. Nano Lett. 2023. PMID: 37363812
-
A caspase-3-activatable bimodal probe for photoacoustic and magnetic resonance imaging of tumor apoptosis in vivo.Biosens Bioelectron. 2022 Nov 15;216:114648. doi: 10.1016/j.bios.2022.114648. Epub 2022 Aug 22. Biosens Bioelectron. 2022. PMID: 36055132
-
Caspase-3-Responsive Dual-Enhanced 1H/19F MRI Bimodal Probe for In Vivo Tumor Apoptosis Imaging.Anal Chem. 2025 May 6;97(17):9265-9275. doi: 10.1021/acs.analchem.4c06853. Epub 2025 Apr 22. Anal Chem. 2025. PMID: 40261975
-
Redox-triggered self-assembly of gadolinium-based MRI probes for sensing reducing environment.Bioconjug Chem. 2014 Aug 20;25(8):1526-36. doi: 10.1021/bc500254g. Epub 2014 Jul 21. Bioconjug Chem. 2014. PMID: 24992373 Free PMC article.
-
Gadolinium-based bimodal probes to enhance T1-Weighted magnetic resonance/optical imaging.Acta Biomater. 2020 Jul 1;110:15-36. doi: 10.1016/j.actbio.2020.03.047. Epub 2020 Apr 23. Acta Biomater. 2020. PMID: 32335310 Review.
Cited by
-
Activated molecular probes for enzyme recognition and detection.Theranostics. 2022 Jan 1;12(3):1459-1485. doi: 10.7150/thno.66676. eCollection 2022. Theranostics. 2022. PMID: 35154500 Free PMC article. Review.
-
Smart Molecular Imaging and Theranostic Probes by Enzymatic Molecular In Situ Self-Assembly.JACS Au. 2024 Jul 3;4(7):2426-2450. doi: 10.1021/jacsau.4c00392. eCollection 2024 Jul 22. JACS Au. 2024. PMID: 39055152 Free PMC article. Review.
-
Bioengineering tools for probing intracellular events in T lymphocytes.WIREs Mech Dis. 2021 Jul;13(4):e1510. doi: 10.1002/wsbm.1510. Epub 2020 Oct 19. WIREs Mech Dis. 2021. PMID: 33073545 Free PMC article. Review.
-
Advances in gadolinium-based MRI contrast agent designs for monitoring biological processes in vivo.Curr Opin Chem Biol. 2018 Aug;45:121-130. doi: 10.1016/j.cbpa.2018.04.006. Epub 2018 May 9. Curr Opin Chem Biol. 2018. PMID: 29751253 Free PMC article. Review.
-
Recent development of nanoparticles for molecular imaging.Philos Trans A Math Phys Eng Sci. 2017 Nov 28;375(2107):20170022. doi: 10.1098/rsta.2017.0022. Philos Trans A Math Phys Eng Sci. 2017. PMID: 29038377 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

