K2CO3-mediated synthesis of functionalised 4-substituted-2-amino-3-cyano-4H-chromenes via Michael-cyclization reactions
- PMID: 25429557
- PMCID: PMC6270755
- DOI: 10.3390/molecules191219253
K2CO3-mediated synthesis of functionalised 4-substituted-2-amino-3-cyano-4H-chromenes via Michael-cyclization reactions
Abstract
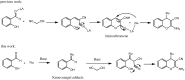
An efficient approach for the synthesis of functionalized 4-substituted-2-amino-3-cyano-4H-chromenes moderate to high yields (up to 98%) has been achieved via a tandem K2CO3 catalyzed conjugate addition-cyclization reaction of malononitrile and a range of Knoevenagel adducts previously formed from oxindole, pyrazolone, nitromethane, N,N-dimethylbarbituric acid or indanedione. This methodology differs from the previous classical methods in its simplicity and ready availability of the catalyst.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Asymmetric Michael addition/intramolecular cyclization catalyzed by bifunctional tertiary amine-squaramides: construction of chiral 2-amino-4H-chromene-3-carbonitrile derivatives.Chem Asian J. 2014 Oct;9(10):2970-4. doi: 10.1002/asia.201402567. Epub 2014 Aug 14. Chem Asian J. 2014. PMID: 25123095
-
Synthesis of functionalized 2-aryl-4-(indol-3-yl)-4H-chromenes via iodine-catalyzed domino Michael addition-intramolecular cyclization reaction.Org Biomol Chem. 2012 Nov 28;10(44):8877-83. doi: 10.1039/c2ob26642c. Org Biomol Chem. 2012. PMID: 23051946
-
Asymmetric synthesis of spiro[4H-chromene-3,3'-oxindoles] via a squaramide-organocatalytic three-component cascade Knoevenagel/Michael/cyclization sequence.Mol Divers. 2025 Feb;29(1):293-302. doi: 10.1007/s11030-024-10852-6. Epub 2024 Apr 30. Mol Divers. 2025. PMID: 38687399
-
Synthesis and pharmacological properties of polysubstituted 2-amino-4H-pyran-3-carbonitrile derivatives.Mol Divers. 2020 Nov;24(4):1385-1431. doi: 10.1007/s11030-019-09994-9. Epub 2019 Sep 25. Mol Divers. 2020. PMID: 31555954 Review.
-
Microwave-assisted synthesis of chromenes: biological and chemical importance.Future Med Chem. 2015;7(7):893-909. doi: 10.4155/fmc.15.38. Future Med Chem. 2015. PMID: 26061107 Review.
Cited by
-
A supramolecular magnetic and multifunctional Titriplex V-grafted chitosan organocatalyst for the synthesis of acridine-1,8-diones and 2-amino-3-cyano-4H-pyran derivatives.Nanoscale Adv. 2024 Oct 2;7(1):99-123. doi: 10.1039/d4na00264d. Online ahead of print. Nanoscale Adv. 2024. PMID: 39502107 Free PMC article.
References
-
- Kjer J., Wray V., Edrada-Ebel R., Ebel R., Pretsch A., Lin W., Proksch P. Xanalteric acids I and II and related phenolic compounds from an endophytic Alternaria sp. Isolated from the mangrove plant Sonneratia alba. J. Nat. Prod. 2006;72:2053–2057. - PubMed
-
- Schmidt T.J., Khalid S.A., Romanha A.J., Alves T.M., Biavatti M.W., Brun R., da Costa F.B., de Castro S.L., Ferreira V.F., de Lacerda M.V., et al. The potential of secondary metabolites from plants as drugs or leads against protozoan neglected diseases-part II. Curr. Med. Chem. 2012;19:2176–2228. - PubMed
-
- Raj T., Bhatia R.K., Kapur A., Sharma M., Saxena A.K., Ishar M.P. CytotoXic activity of 3-(5-phenyl-3H-[1,2,4]dithiazol-3-yl)chromen-4-ones and 4-oxo-4H-chromene-3-carbothioic acid N-phenylamides. Eur. J. Med. Chem. 2010;45:790–794. - PubMed
-
- Mungra D.C., Patel M.P., Rajani D.P., Patel R.G. Synthesis and identification of β-aryloxyquinolines and their pyrano[3,3-c]chromene derivatives as a new class of antimicrobial and antituberculosis agents. Eur. J. Med. Chem. 2011;46:4192–4200. - PubMed
-
- Conti C., Proietti Monaco L., Desideri N. Synthesis and anti-rhinovirus activity of novel 3-[2-(pyridinyl)vinyl]substituted-2H-chromenes and 4H-chromen-4-ones. Bioorg. Med. Chem. 2014;22:1201–1207. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical