Transforming growth factor-beta 3 alters intestinal smooth muscle function: implications for gastroschisis-related intestinal dysfunction
- PMID: 25431043
- PMCID: PMC4427617
- DOI: 10.1007/s10620-014-3439-1
Transforming growth factor-beta 3 alters intestinal smooth muscle function: implications for gastroschisis-related intestinal dysfunction
Abstract
Background: Gastroschisis (GS) is a congenital abdominal wall defect that results in the development of GS-related intestinal dysfunction (GRID). Transforming growth factor-β, a pro-inflammatory cytokine, has been shown to cause organ dysfunction through alterations in vascular and airway smooth muscle. The purpose of this study was to evaluate the effects of TGF-β3 on intestinal smooth muscle function and contractile gene expression.
Methods: Archived human intestinal tissue was analyzed using immunohistochemistry and RT-PCR for TGF-β isoforms and markers of smooth muscle gene and micro-RNA contractile phenotype. Intestinal motility was measured in neonatal rats ± TGF-β3 (0.2 and 1 mg/kg). Human intestinal smooth muscle cells (hiSMCs) were incubated with fetal bovine serum ± 100 ng/ml of TGF-β 3 isoforms for 6, 24 and 72 h. The effects of TGF-β3 on motility, hiSMC contractility and hiSMC contractile phenotype gene and micro-RNA expression were measured using transit, collagen gel contraction assay and RT-PCR analysis. Data are expressed as mean ± SEM, ANOVA (n = 6-7/group).
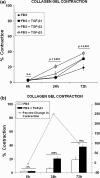
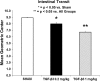
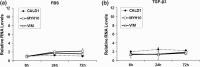
Results: GS infants had increased immunostaining of TGF-β3 and elevated levels of micro-RNA 143 & 145 in the intestinal smooth muscle. Rats had significantly decreased intestinal transit when exposed to TGF-β3 in a dose-dependent manner compared with Sham animals. TGF-β3 significantly increased hiSMC gel contraction and contractile protein gene and micro-RNA expression.
Conclusion: TGF-β3 contributed to intestinal dysfunction at the organ level, increased contraction at the cellular level and elevated contractile gene expression at the molecular level. A hyper-contractile response may play a role in the persistent intestinal dysfunction seen in GRID.
Figures



Similar articles
-
Interferon-gamma depresses human intestinal smooth muscle cell contractility: Relevance to inflammatory gut motility disturbances.Life Sci. 2019 Apr 1;222:69-77. doi: 10.1016/j.lfs.2019.01.059. Epub 2019 Feb 27. Life Sci. 2019. PMID: 30825545
-
[Expression of cellular phenotype switching markers-matrix protein Gla, mRNA and collagen I, III and V of human airway smooth muscle cells in vitro after TGF-beta1 stimulation].Zhonghua Er Ke Za Zhi. 2006 Jul;44(7):531-4. Zhonghua Er Ke Za Zhi. 2006. PMID: 17044981 Chinese.
-
Assessing the effects of transforming growth factor-beta1 on bladder smooth muscle cell phenotype. I. Modulation of in vitro contractility.J Urol. 2009 Sep;182(3):1210-5. doi: 10.1016/j.juro.2009.05.002. Epub 2009 Jul 21. J Urol. 2009. PMID: 19625042
-
Mechanism of abnormal intestinal motility in inflammatory bowel disease: how smooth muscle contraction is reduced?J Smooth Muscle Res. 2007 Apr;43(2):43-54. doi: 10.1540/jsmr.43.43. J Smooth Muscle Res. 2007. PMID: 17598957 Review.
-
Reevaluation of Pluripotent Cytokine TGF-β3 in Immunity.Int J Mol Sci. 2018 Aug 1;19(8):2261. doi: 10.3390/ijms19082261. Int J Mol Sci. 2018. PMID: 30071700 Free PMC article. Review.
Cited by
-
Bioengineered intestinal muscularis complexes with long-term spontaneous and periodic contractions.PLoS One. 2018 May 2;13(5):e0195315. doi: 10.1371/journal.pone.0195315. eCollection 2018. PLoS One. 2018. PMID: 29718926 Free PMC article.
-
MicroRNA Regulatory Pathways in the Control of the Actin-Myosin Cytoskeleton.Cells. 2020 Jul 9;9(7):1649. doi: 10.3390/cells9071649. Cells. 2020. PMID: 32660059 Free PMC article. Review.
-
Differences in proliferation rate between CADASIL and control vascular smooth muscle cells are related to increased TGFβ expression.J Cell Mol Med. 2018 Jun;22(6):3016-3024. doi: 10.1111/jcmm.13534. Epub 2018 Mar 13. J Cell Mol Med. 2018. PMID: 29536621 Free PMC article.
-
miRNA-143 expression is associated with inflammation and time of exposure to amniotic fluid in experimental gastroschisis.Clinics (Sao Paulo). 2023 Nov 25;78:100311. doi: 10.1016/j.clinsp.2023.100311. eCollection 2023. Clinics (Sao Paulo). 2023. PMID: 38008037 Free PMC article.
-
The Role of Inflammatory Mediators in the Development of Gastrointestinal Motility Disorders.Int J Mol Sci. 2022 Jun 22;23(13):6917. doi: 10.3390/ijms23136917. Int J Mol Sci. 2022. PMID: 35805922 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

