Enterococcus faecalis pCF10-encoded surface proteins PrgA, PrgB (aggregation substance) and PrgC contribute to plasmid transfer, biofilm formation and virulence
- PMID: 25431047
- PMCID: PMC4329047
- DOI: 10.1111/mmi.12893
Enterococcus faecalis pCF10-encoded surface proteins PrgA, PrgB (aggregation substance) and PrgC contribute to plasmid transfer, biofilm formation and virulence
Abstract
Enterococcus faecalis pCF10 transfers at high frequencies upon pheromone induction of the prgQ transfer operon. This operon codes for three cell wall-anchored proteins - PrgA, PrgB (aggregation substance) and PrgC - and a type IV secretion system through which the plasmid is delivered to recipient cells. Here, we defined the contributions of the Prg surface proteins to plasmid transfer, biofilm formation and virulence using the Caenorhabditis elegans infection model. We report that a combination of PrgB and extracellular DNA (eDNA), but not PrgA or PrgC, was required for extensive cellular aggregation and pCF10 transfer at wild-type frequencies. In addition to PrgB and eDNA, production of PrgA was necessary for extensive binding of enterococci to abiotic surfaces and development of robust biofilms. However, although PrgB is a known virulence factor in mammalian infection models, we determined that PrgA and PrgC, but not PrgB, were required for efficient killing in the worm infection model. We propose that the pheromone-responsive, conjugative plasmids of E. faecalis have retained Prg-like surface functions over evolutionary time for attachment, colonization and robust biofilm development. In natural settings, these biofilms are polymicrobial in composition and constitute optimal environments for signal exchange, mating pair formation and widespread lateral gene transfer.
© 2014 John Wiley & Sons Ltd.
Figures

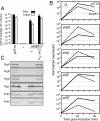

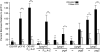


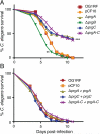
Similar articles
-
PrgU: a suppressor of sex pheromone toxicity in Enterococcus faecalis.Mol Microbiol. 2017 Feb;103(3):398-412. doi: 10.1111/mmi.13563. Epub 2016 Dec 16. Mol Microbiol. 2017. PMID: 27785854 Free PMC article.
-
Genetic analysis of a region of the Enterococcus faecalis plasmid pCF10 involved in positive regulation of conjugative transfer functions.J Bacteriol. 1995 Apr;177(8):2107-17. doi: 10.1128/jb.177.8.2107-2117.1995. J Bacteriol. 1995. PMID: 7721703 Free PMC article.
-
Transcriptional analysis of a region of the Enterococcus faecalis plasmid pCF10 involved in positive regulation of conjugative transfer functions.J Bacteriol. 1995 Apr;177(8):2118-24. doi: 10.1128/jb.177.8.2118-2124.1995. J Bacteriol. 1995. PMID: 7536732 Free PMC article.
-
The peptide pheromone-inducible conjugation system of Enterococcus faecalis plasmid pCF10: cell-cell signalling, gene transfer, complexity and evolution.Philos Trans R Soc Lond B Biol Sci. 2007 Jul 29;362(1483):1185-93. doi: 10.1098/rstb.2007.2043. Philos Trans R Soc Lond B Biol Sci. 2007. PMID: 17360276 Free PMC article. Review.
-
The sex pheromone system of Enterococcus faecalis. More than just a plasmid-collection mechanism?Eur J Biochem. 1994 Jun 1;222(2):235-46. doi: 10.1111/j.1432-1033.1994.tb18862.x. Eur J Biochem. 1994. PMID: 8020463 Review.
Cited by
-
PrgU: a suppressor of sex pheromone toxicity in Enterococcus faecalis.Mol Microbiol. 2017 Feb;103(3):398-412. doi: 10.1111/mmi.13563. Epub 2016 Dec 16. Mol Microbiol. 2017. PMID: 27785854 Free PMC article.
-
Pheromone Activity after Stimulation with Ampicillin in a Plasmid-Free Enterococcus faecalis Strain.Microorganisms. 2022 Nov 19;10(11):2294. doi: 10.3390/microorganisms10112294. Microorganisms. 2022. PMID: 36422364 Free PMC article.
-
Factors affecting CRISPR-Cas defense against antibiotic resistance plasmids harbored by Enterococcus faecalis laboratory model strains and clinical isolates.bioRxiv [Preprint]. 2025 Jul 1:2025.03.10.642232. doi: 10.1101/2025.03.10.642232. bioRxiv. 2025. PMID: 40161755 Free PMC article. Preprint.
-
Examination of Enterococcus faecalis Toxin-Antitoxin System Toxin Fst Function Utilizing a Pheromone-Inducible Expression Vector with Tight Repression and Broad Dynamic Range.J Bacteriol. 2017 May 25;199(12):e00065-17. doi: 10.1128/JB.00065-17. Print 2017 Jun 15. J Bacteriol. 2017. PMID: 28348028 Free PMC article.
-
Involvement of Chromosomally Encoded Homologs of the RRNPP Protein Family in Enterococcus faecalis Biofilm Formation and Urinary Tract Infection Pathogenesis.J Bacteriol. 2020 Aug 10;202(17):e00063-20. doi: 10.1128/JB.00063-20. Print 2020 Aug 10. J Bacteriol. 2020. PMID: 32540933 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01GM48746/GM/NIGMS NIH HHS/United States
- R21AI105454/AI/NIAID NIH HHS/United States
- R01GM49530/GM/NIGMS NIH HHS/United States
- R01 GM049530/GM/NIGMS NIH HHS/United States
- R56 AI110432/AI/NIAID NIH HHS/United States
- R01 GM048746/GM/NIGMS NIH HHS/United States
- R21 AI105454/AI/NIAID NIH HHS/United States
- R01AI076406/AI/NIAID NIH HHS/United States
- R01 AI076406/AI/NIAID NIH HHS/United States
- R56AI110432/AI/NIAID NIH HHS/United States
- R01DE021394/DE/NIDCR NIH HHS/United States
- R01 DE021394/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

