Protocol for the Femoroacetabular Impingement Trial (FAIT): a multi-centre randomised controlled trial comparing surgical and non-surgical management of femoroacetabular impingement
- PMID: 25431439
- PMCID: PMC4248299
- DOI: 10.1302/2046-3758.311.2000336
Protocol for the Femoroacetabular Impingement Trial (FAIT): a multi-centre randomised controlled trial comparing surgical and non-surgical management of femoroacetabular impingement
Abstract
Aims: Femoroacetabular Junction Impingement (FAI) describes abnormalities in the shape of the femoral head-neck junction, or abnormalities in the orientation of the acetabulum. In the short term, FAI can give rise to pain and disability, and in the long-term it significantly increases the risk of developing osteoarthritis. The Femoroacetabular Impingement Trial (FAIT) aims to determine whether operative or non-operative intervention is more effective at improving symptoms and preventing the development and progression of osteoarthritis.
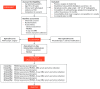
Methods: FAIT is a multicentre superiority parallel two-arm randomised controlled trial comparing physiotherapy and activity modification with arthroscopic surgery for the treatment of symptomatic FAI. Patients aged 18 to 60 with clinical and radiological evidence of FAI are eligible. Principal exclusion criteria include previous surgery to the index hip, established osteoarthritis (Kellgren-Lawrence ≥ 2), hip dysplasia (centre-edge angle < 20°), and completion of a physiotherapy programme targeting FAI within the previous 12 months. Recruitment will take place over 24 months and 120 patients will be randomised in a 1:1 ratio and followed up for three years. The two primary outcome measures are change in hip outcome score eight months post-randomisation (approximately six-months post-intervention initiation) and change in radiographic minimum joint space width 38 months post-randomisation. ClinicalTrials.gov: NCT01893034. Cite this article: Bone Joint Res 2014;3:321-7.
Keywords: Femoroacetabular Impingment; Hip Arthroscopy; Osteoarthritis; Physiotherapy; Randomised Controlled Trial.
©2014 The British Editorial Society of Bone & Joint Surgery.
Conflict of interest statement
Figures
References
-
- Ganz R, Parvizi J, Beck M, et al. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res 2003;417:112–120. - PubMed
-
- Laborie LB, Lehmann TG, Engesæter IØ, et al. Prevalence of radiographic findings thought to be associated with femoroacetabular impingement in a population-based cohort of 2081 healthy young adults. Radiology 2011;260:494–502. - PubMed
-
- Agricola R, Waarsing JH, Arden NK. , et al. Cam impingement of the hip: a risk factor for hip osteoarthritis. Nat Rev Rheumatol 2013;9:630–634. - PubMed
-
- Khanna V, Caragianis A, Diprimio G, Rakhra K, Beaulé PE. Incidence of hip pain in a prospective cohort of asymptomatic volunteers: is the cam deformity a risk factor for hip pain? Am J Sports Med 2014;42:793–797. - PubMed
-
- Agricola R, Heijboer MP, Bierma-Zeinstra SM, et al. Cam impingement causes osteoarthritis of the hip: a nationwide prospective cohort study (CHECK). Ann Rheum Dis 2013;72:918–923. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical