A (Fluoroalkyl)Guanidine Modulates the Relaxivity of a Phosphonate-Containing T1-Shortening Contrast Agent
- PMID: 25431503
- PMCID: PMC4241975
- DOI: 10.1016/j.jfluchem.2014.09.018
A (Fluoroalkyl)Guanidine Modulates the Relaxivity of a Phosphonate-Containing T1-Shortening Contrast Agent
Abstract
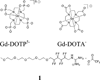
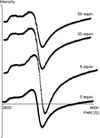
Responsive magnetic resonance imaging (MRI) contrast agents, those that change their relaxivity according to environmental stimuli, have promise as next generation imaging probes in medicine. While several of these are known based on covalent modification of the contrast agents, fewer are known based on controlling non-covalent interactions. We demonstrate here accentuated relaxivity of a T1-shortening contrast agent, Gd-DOTP5- based on non-covalent, hydrogen bonding of Gd-DOTP5- with a novel fluorous amphiphile. By contrast to the phosphonate-containing Gd-DOTP5- system, the relaxivity of the analogous clinically approved contrast agent, Gd-DOTA- is unaffected by the same fluorous amphiphile under similar conditions. Mechanistic studies show that placing the fluorous amphiphile in proximity of the gadolinium center in Gd-DOTP5- caused an increase in τ m (bound-water residence lifetime or the inverse of water exchange rate, τ m = 1/kex) and an increase in τ R (rotational correlation time), with τ R being the factor driving enhanced relaxivity. Further, these effects were not observed when Gd-DOTA- was treated with the same fluorous amphiphile. Thus, Gd-DOTP5- and Gd-DOTA- respond to the fluorous amphiphile differently, presumably because the former binds to the amphiphile with higher affinity. (DOTP = 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraphosphonic acid; DOTA = 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid).
Keywords: DOTP; Fluoroakyl Groups; Gadolinium; MRI Contrast; T1.
Figures
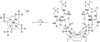
Similar articles
-
The Gd(3+) complex of a fatty acid analogue of DOTP binds to multiple albumin sites with variable water relaxivities.Inorg Chem. 2001 Dec 17;40(26):6580-7. doi: 10.1021/ic0102900. Inorg Chem. 2001. PMID: 11735466
-
Synthesis, potentiometric, kinetic, and NMR Studies of 1,4,7,10-tetraazacyclododecane-1,7-bis(acetic acid)-4,10-bis(methylenephosphonic acid) (DO2A2P) and its complexes with Ca(II), Cu(II), Zn(II) and lanthanide(III) ions.Inorg Chem. 2008 May 5;47(9):3851-62. doi: 10.1021/ic7024704. Epub 2008 Apr 2. Inorg Chem. 2008. PMID: 18380456
-
Brain tumor enhancement in magnetic resonance imaging at 3 tesla: intraindividual comparison of two high relaxivity macromolecular contrast media with a standard extracellular gd-chelate in a rat brain tumor model.Invest Radiol. 2009 Apr;44(4):200-6. doi: 10.1097/RLI.0b013e31819817ff. Invest Radiol. 2009. PMID: 19300099
-
Cyclen-based Gd3+ complexes as MRI contrast agents: Relaxivity enhancement and ligand design.Bioorg Med Chem. 2016 Nov 15;24(22):5663-5684. doi: 10.1016/j.bmc.2016.09.069. Epub 2016 Oct 1. Bioorg Med Chem. 2016. PMID: 27729196 Review.
-
Gadolinium-based bimodal probes to enhance T1-Weighted magnetic resonance/optical imaging.Acta Biomater. 2020 Jul 1;110:15-36. doi: 10.1016/j.actbio.2020.03.047. Epub 2020 Apr 23. Acta Biomater. 2020. PMID: 32335310 Review.
Cited by
-
Preparation of MnO2@poly-(DMAEMA-co-IA)-conjugated methotrexate nano-complex for MRI and radiotherapy of breast cancer application.MAGMA. 2023 Oct;36(5):779-795. doi: 10.1007/s10334-023-01091-1. Epub 2023 Apr 19. MAGMA. 2023. PMID: 37074514
-
pH-responsive i-motif-conjugated nanoparticles for MRI analysis.Sens Diagn. 2024 Mar 6;3(4):623-630. doi: 10.1039/d3sd00285c. eCollection 2024 Apr 18. Sens Diagn. 2024. PMID: 38646186 Free PMC article.
-
Aqueous Lanthanide Chemistry in Asymmetric Catalysis and Magnetic Resonance Imaging.Synlett. 2016 Jun;27(9):1310-1317. doi: 10.1055/s-0035-1561363. Synlett. 2016. PMID: 27493448 Free PMC article.
-
Nano assembly and encapsulation; a versatile platform for slowing the rotation of polyanionic Gd(3+) -based MRI contrast agents.Contrast Media Mol Imaging. 2016 Mar-Apr;11(2):154-9. doi: 10.1002/cmmi.1676. Epub 2015 Dec 28. Contrast Media Mol Imaging. 2016. PMID: 26708733 Free PMC article.
-
Functionalized Gold Nanoparticles as Contrast Agents for Proton and Dual Proton/Fluorine MRI.Nanomaterials (Basel). 2019 Jun 13;9(6):879. doi: 10.3390/nano9060879. Nanomaterials (Basel). 2019. PMID: 31200518 Free PMC article.
References
-
- González G, Powell DH, Tissières V, Merbach AE. J. Phys. Chem. 1994;98:53–59.
- Powell HP, Ni Dhubhghail OM, Pubanz D, Helm L, Lebedev YS, Schlaepfer W, Merbach AE. J. Am. Chem. Soc. 1996;118:9333–9346.
-
- Merbach AS, Helm L, Tóth É. The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging. 2nd ed. New York: John Wiley & Sons; 2013.
-
-
a. Contrast Agents I: Magnetic Resonance Imaging, Krause W. Topics in Current Chemistry. New York: Springer-Verlag; 2002. Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Chem. Rev. 1999;99:2293–2352. Aime S, Botta M, Fasano M, Terreno E. Chem. Soc. Rev. 1998;27:19–29. Manus LM, Strauch RC, Hung AH, Eckermann AL, Meade TJ. Anal. Chem. 2012;84:6278–6287.
-
-
- Moats, Fraser RA, Meade SE, Angew TJ. Chem. Int. Ed. 1997;36:726–728.
- Stavila V, Allali M, Canaple L, Stortz Y, Franc C, Marin P, Beuf O, Dufay O, Samarut J, Janier M, Hasserodt J. New J. Chem. 2008;32:428–435.
-
- Que EL, Gianolio E, Baker SL, Wong AP, Aime S, Chang CJ. J. Am. Chem. Soc. 2009;131:8527–8536. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
