Intermingled cAMP, cGMP and calcium spatiotemporal dynamics in developing neuronal circuits
- PMID: 25431549
- PMCID: PMC4230202
- DOI: 10.3389/fncel.2014.00376
Intermingled cAMP, cGMP and calcium spatiotemporal dynamics in developing neuronal circuits
Abstract
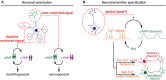
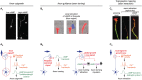
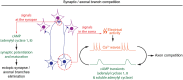
cAMP critically modulates the development of neuronal connectivity. It is involved in a wide range of cellular processes that require independent regulation. However, our understanding of how this single second messenger achieves specific modulation of the signaling pathways involved remains incomplete. The subcellular compartmentalization and temporal regulation of cAMP signals have recently been identified as important coding strategies leading to specificity. Dynamic interactions of this cyclic nucleotide with other second messenger including calcium and cGMP are critically involved in the regulation of spatiotemporal control of cAMP. Recent technical improvements of fluorescent sensors facilitate cAMP monitoring, whereas optogenetic tools permit spatial and temporal control of cAMP manipulations, all of which enabled the direct investigation of spatiotemporal characteristics of cAMP modulation in developing neurons. Focusing on neuronal polarization, neurotransmitter specification, axon guidance, and refinement of neuronal connectivity, we summarize herein the recent advances in understanding the features of cAMP signals and their dynamic interactions with calcium and cGMP involved in shaping the nervous system.
Keywords: axon guidance; axon outgrowth; cAMP; cGMP; calcium; kinetics; subcellular compartmentalization; topographic maps.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

