A Comparative View on Human Somatic Cell Sources for iPSC Generation
- PMID: 25431601
- PMCID: PMC4241335
- DOI: 10.1155/2014/768391
A Comparative View on Human Somatic Cell Sources for iPSC Generation
Abstract
The breakthrough of reprogramming human somatic cells was achieved in 2006 by the work of Yamanaka and Takahashi. From this point, fibroblasts are the most commonly used primary somatic cell type for the generation of induced pluripotent stem cells (iPSCs). Various characteristics of fibroblasts supported their utilization for the groundbreaking experiments of iPSC generation. One major advantage is the high availability of fibroblasts which can be easily isolated from skin biopsies. Furthermore, their cultivation, propagation, and cryoconservation properties are uncomplicated with respect to nutritional requirements and viability in culture. However, the required skin biopsy remains an invasive approach, representing a major drawback for using fibroblasts as the starting material. More and more studies appeared over the last years, describing the reprogramming of other human somatic cell types. Cells isolated from blood samples or urine, as well as more unexpected cell types, like pancreatic islet beta cells, synovial cells, or mesenchymal stromal cells from wisdom teeth, show promising characteristics for a reprogramming strategy. Here, we want to highlight the advantages of keratinocytes from human plucked hair as a widely usable, noninvasive harvesting method for primary material in comparison with other commonly used cell types.
Figures
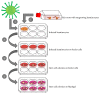
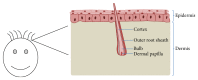

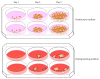
References
-
- Maetzel D., Sarkar S., Wang H., Abi-Mosleh L., Xu P., Cheng A. W., Gao Q., Mitalipova M., Jaenisch R. Genetic and chemical correction of cholesterol accumulation and impaired autophagy in hepatic and neural cells derived from Niemann-Pick type C patient-specific iPS cells. Stem Cell Reports. 2014;2(6):866–880. - PMC - PubMed
-
- Reinhardt P., Schmid B., Burbulla L. F., Schöndorf D. C., Wagner L., Glatza M., Höing S., Hargus G., Heck S. A., Dhingra A., Wu G., Müller S., Brockmann K., Kluba T., Maisel M., Krüger R., Berg D., Tsytsyura Y., Thiel C. S., Psathaki O.-E., Klingauf J., Kuhlmann T., Klewin M., Müller H., Gasser T., Schöler H. R., Sterneckert J. Genetic correction of a lrrk2 mutation in human iPSCs links parkinsonian neurodegeneration to ERK-dependent changes in gene expression. Cell Stem Cell. 2013;12(3):354–367. doi: 10.1016/j.stem.2013.01.008. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

