Plasma and Intracellular Antiretroviral Concentrations in HIV-Infected Patients under Short Cycles of Antiretroviral Therapy
- PMID: 25431661
- PMCID: PMC4241735
- DOI: 10.1155/2014/724958
Plasma and Intracellular Antiretroviral Concentrations in HIV-Infected Patients under Short Cycles of Antiretroviral Therapy
Abstract
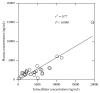
Study of plasma and intracellular concentrations of atazanavir, lopinavir, nevirapine, and efavirenz was conducted on 48 patients under short cycles of antiretroviral therapy. Intracellular concentrations (IC) were still measurable for all drugs after 85 h or 110 h drug intake despite the absence of drug in plasma for atazanavir and lopinavir. A linear relationship between plasma and intracellular efavirenz was observed. Further studies to fully understand the impact of IC in the intermittent antiviral treatment are required.
Figures
References
-
- Azoulay S., Nevers M.-C., Créminon C., Heripret L., Garraffo R., Durant J., Dellamonica P., Grassi J., Guedj R., Duval D. An enzyme immunoassay for the quantification of plasma and intracellular lopinavir in HIV-infected patients. Journal of Immunological Methods. 2004;295(1-2):37–48. doi: 10.1016/j.jim.2004.08.016. - DOI - PubMed
-
- Azoulay S., Nevers M.-C., Créminon C., Heripret L., Durant J., Dellamonica P., Grassi J., Guedj R., Duval D. Sensitive enzyme immunoassay for measuring plasma and intracellular nevirapine levels in human immunodeficiency virus-infected patients. Antimicrobial Agents and Chemotherapy. 2004;48(1):104–109. doi: 10.1128/AAC.48.1.104-109.2004. - DOI - PMC - PubMed
-
- Roucairol C., Azoulay S., Nevers M.-C., Créminon C., Lavrut T., Garraffo R., Grassi J., Burger A., Duval D. Quantitative immunoassay to measure plasma and intracellular atazanavir levels: analysis of drug accumulation in cultured T cells. Antimicrobial Agents and Chemotherapy. 2007;51(2):405–411. doi: 10.1128/AAC.00730-06. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical