Phosphatidylinositol 3-kinase and COPII generate LC3 lipidation vesicles from the ER-Golgi intermediate compartment
- PMID: 25432021
- PMCID: PMC4270069
- DOI: 10.7554/eLife.04135
Phosphatidylinositol 3-kinase and COPII generate LC3 lipidation vesicles from the ER-Golgi intermediate compartment
Abstract
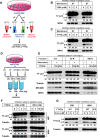
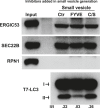
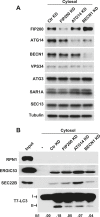
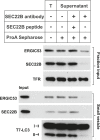
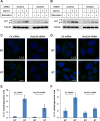
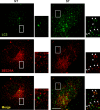
Formation of the autophagosome requires significant membrane input from cellular organelles. However, no direct evidence has been developed to link autophagic factors and the mobilization of membranes to generate the phagophore. Previously, we established a cell-free LC3 lipidation reaction to identify the ER-Golgi intermediate compartment (ERGIC) as a membrane source for LC3 lipidation, a key step of autophagosome biogenesis (Ge et al., eLife 2013; 2:e00947). We now report that starvation activation of autophagic phosphotidylinositol-3 kinase (PI3K) induces the generation of small vesicles active in LC3 lipidation. Subcellular fractionation studies identified the ERGIC as the donor membrane in the generation of small lipidation-active vesicles. COPII proteins are recruited to the ERGIC membrane in starved cells, dependent on active PI3K. We conclude that starvation activates the autophagic PI3K, which in turn induces the recruitment of COPII to the ERGIC to bud LC3 lipidation-active vesicles as one potential membrane source of the autophagosome.
Keywords: COPII; ER-Golgi intermediate compartment; LC3 lipidation; Phosphatidylinositol 3-kinase; autophagosome; autophagy; biochemistry; cell biology; human; mouse.
Conflict of interest statement
RS: Editor in Chief,
The other authors declare that no competing interests exist.
Figures








Similar articles
-
Biogenesis of autophagosomal precursors for LC3 lipidation from the ER-Golgi intermediate compartment.Autophagy. 2015;11(12):2372-4. doi: 10.1080/15548627.2015.1105422. Autophagy. 2015. PMID: 26565421 Free PMC article. Review.
-
Remodeling of ER-exit sites initiates a membrane supply pathway for autophagosome biogenesis.EMBO Rep. 2017 Sep;18(9):1586-1603. doi: 10.15252/embr.201744559. Epub 2017 Jul 28. EMBO Rep. 2017. PMID: 28754694 Free PMC article.
-
The ER-Golgi intermediate compartment feeds the phagophore membrane.Autophagy. 2014 Jan;10(1):170-2. doi: 10.4161/auto.26787. Epub 2013 Nov 11. Autophagy. 2014. PMID: 24220263 Free PMC article.
-
The ER-Golgi intermediate compartment is a key membrane source for the LC3 lipidation step of autophagosome biogenesis.Elife. 2013 Aug 6;2:e00947. doi: 10.7554/eLife.00947. Elife. 2013. PMID: 23930225 Free PMC article.
-
Multifaceted roles of COPII subunits in autophagy.Biochim Biophys Acta Mol Cell Res. 2020 Apr;1867(4):118627. doi: 10.1016/j.bbamcr.2019.118627. Epub 2019 Dec 19. Biochim Biophys Acta Mol Cell Res. 2020. PMID: 31863790 Review.
Cited by
-
ER exit in physiology and disease.Front Mol Biosci. 2024 Jan 18;11:1352970. doi: 10.3389/fmolb.2024.1352970. eCollection 2024. Front Mol Biosci. 2024. PMID: 38314136 Free PMC article. Review.
-
The endolysosomal system in conventional and unconventional protein secretion.J Cell Biol. 2024 Sep 2;223(9):e202404152. doi: 10.1083/jcb.202404152. Epub 2024 Aug 12. J Cell Biol. 2024. PMID: 39133205 Free PMC article. Review.
-
Microglia ferroptosis is regulated by SEC24B and contributes to neurodegeneration.Nat Neurosci. 2023 Jan;26(1):12-26. doi: 10.1038/s41593-022-01221-3. Epub 2022 Dec 19. Nat Neurosci. 2023. PMID: 36536241 Free PMC article.
-
Role of autophagy in IL-1β export and release from cells.Semin Cell Dev Biol. 2018 Nov;83:36-41. doi: 10.1016/j.semcdb.2018.03.012. Epub 2018 Apr 5. Semin Cell Dev Biol. 2018. PMID: 29580970 Free PMC article. Review.
-
TRIM proteins in autophagy: selective sensors in cell damage and innate immune responses.Cell Death Differ. 2020 Mar;27(3):887-902. doi: 10.1038/s41418-020-0495-2. Epub 2020 Jan 22. Cell Death Differ. 2020. PMID: 31969691 Free PMC article. Review.
References
-
- Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A, Griffiths G, Ktistakis NT. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. The Journal of Cell Biology. 2008;182:685–701. doi: 10.1083/jcb.200803137. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

