Disruption of thalamic functional connectivity is a neural correlate of dexmedetomidine-induced unconsciousness
- PMID: 25432022
- PMCID: PMC4280551
- DOI: 10.7554/eLife.04499
Disruption of thalamic functional connectivity is a neural correlate of dexmedetomidine-induced unconsciousness
Abstract
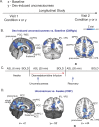
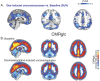
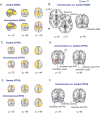
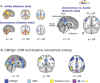
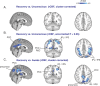
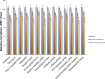
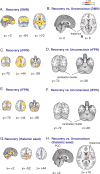
Understanding the neural basis of consciousness is fundamental to neuroscience research. Disruptions in cortico-cortical connectivity have been suggested as a primary mechanism of unconsciousness. By using a novel combination of positron emission tomography and functional magnetic resonance imaging, we studied anesthesia-induced unconsciousness and recovery using the α₂-agonist dexmedetomidine. During unconsciousness, cerebral metabolic rate of glucose and cerebral blood flow were preferentially decreased in the thalamus, the Default Mode Network (DMN), and the bilateral Frontoparietal Networks (FPNs). Cortico-cortical functional connectivity within the DMN and FPNs was preserved. However, DMN thalamo-cortical functional connectivity was disrupted. Recovery from this state was associated with sustained reduction in cerebral blood flow and restored DMN thalamo-cortical functional connectivity. We report that loss of thalamo-cortical functional connectivity is sufficient to produce unconsciousness.
Keywords: consciousness; cortico-cortical; default mode network; dexmedetomidine; functional connectivity; human; neuroscience; thalamo-cortical.
Conflict of interest statement
The author declare that no competing interests exist.
Figures

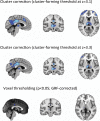
References
-
- Akeju O, Pavone KJ, Westover MB, Vazquez R, Prerau MJ, Harrell PG, Hartnack KE, Rhee J, Sampson AL, Habeeb K, Lei G, Pierce ET, Walsh JL, Brown EN, Purdon PL. A comparison of propofol- and dexmedetomidine-induced electroencephalogram dynamics using Spectral and Coherence analysis. Anesthesiology. 2014;121:978–989. doi: 10.1097/ALN.0000000000000419. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

