RNase E in the γ-Proteobacteria: conservation of intrinsically disordered noncatalytic region and molecular evolution of microdomains
- PMID: 25432321
- PMCID: PMC4435900
- DOI: 10.1007/s00438-014-0959-5
RNase E in the γ-Proteobacteria: conservation of intrinsically disordered noncatalytic region and molecular evolution of microdomains
Abstract
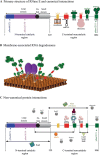
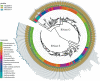
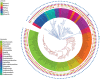
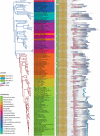
RNase E of Escherichia coli is a membrane-associated endoribonuclease that has a major role in mRNA degradation. The enzyme has a large C-terminal noncatalytic region that is mostly intrinsically disordered (ID). Under standard growth conditions, RhlB, enolase and PNPase associate with the noncatalytic region to form the multienzyme RNA degradosome. To elucidate the origin and evolution of the RNA degradosome, we have identified and characterized orthologs of RNase E in the γ-Proteobacteria, a phylum of bacteria with diverse ecological niches and metabolic phenotypes and an ancient origin contemporary with the radiation of animals, plants and fungi. Intrinsic disorder, composition bias and tandem sequence repeats are conserved features of the noncatalytic region. Composition bias is bipartite with a catalytic domain proximal ANR-rich region and distal AEPV-rich region. Embedded in the noncatalytic region are microdomains (also known as MoRFs, MoREs or SLiMs), which are motifs that interact with protein and other ligands. Our results suggest that tandem repeat sequences are the progenitors of microdomains. We have identified 24 microdomains with phylogenetic signals that were acquired once with few losses. Microdomains involved in membrane association and RNA binding are universally conserved suggesting that they were present in ancestral RNase E. The RNA degradosome of E. coli arose in two steps with RhlB and PNPase acquisition early in a major subtree of the γ-Proteobacteria and enolase acquisition later. We propose a mechanism of microdomain acquisition and evolution and discuss implications of these results for the structure and function of the multienzyme RNA degradosome.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

